Introduction
Instead of a conventional state-of-the-art review, this A to Z dictionary attempts to provide a practical guide for the application of coronary physiology in the catheterisation laboratory (cath lab), exploring several methods, their pitfalls, and useful tips and tricks. Multiple illustrated summaries seek to conduct the interventional cardiologist from the cornerstone of coronary physiology towards recent innovations and augmented reality, attempting to highlight the essential contributions of physiology-based diagnoses and guidance for the personalised management of CAD in the real world.
The format of this review is somewhat unusual, unusual in the medical field at least. There are indeed very successful dictionary collections about literature, art, sport and philosophy. Similar to those, this article is a collection of short pieces classified in alphabetical order, without a linear structure. The review statements are understandable as standalone abstracts and the reader is referenced to connected sections in order to link different concepts with each other (for instance: See ➔ Grey zone). Importantly, much like the capricious “Encyclopaedia of Everything and Nothing”, contributions are not intended to be comprehensive or consensual but rather short essays of a subjective nature providing, at times, opinionated viewpoints on the matter. The authors have also decided to prioritise many unique contributions and important recent advances in research from the East, namely China, Japan and the Republic of Korea. According to our knowledge, this is the first “state-of-the-art medical review” conceptualised as a personal viewpoint in the format of an “A to Z dictionary”.
Angiography
Invasive coronary angiography (ICA) is a minimally invasive procedure that, through the injection of a radio-opaque dye and use of an X-ray machine, creates a beating lumenogram of the epicardial coronary tree, allowing the identification of narrowings (or stenoses) potentially responsible for nutrient flow reduction and myocardial ischaemia in patients with CAD. A 50% diameter stenosis (DS) is used as the anatomically significant cut-off to guide treatment choices between medical treatment and revascularisation. Visual stenosis estimation is inaccurate and poorly reproducible, especially when compared to stenosis-specific functional assessment by fractional flow reserve (FFR) and pressure wires (PW)1. Indeed, FFR is preserved in 65% of 50-70% DS by angiography2. New computational models have been developed to derive the functional significance of a given stenosis without the use of a dedicated PW3. However, ICA with its high spatial (up to 1 mm) and temporal (up to 30 frames/sec) resolution remains the global positioning system (GPS) for the anatomic diagnosis of coronary artery disease (CAD). Its fusion with physiology and/or intracoronary imaging allows the coregistration of anatomy and physiology.
See ➔ Imaging, QFR, Virtual PCI
Acute coronary syndromes
Acute coronary syndromes (ACS) represent the clinical correlates of a sudden reduction of the blood supply to the heart, causing myocardial infarction (MI) or unstable angina. ACS patients often present with bystander multivessel CAD (~50%)4.
Culprit stenosis
The epicardial culprit of MI is most often readily identified on the ICA from clinical symptoms, electrocardiographic changes and wall motion abnormalities. Likewise, in non-ST-segment elevation MI, the role of physiological assessment of the culprit lesion is limited. Imaging the underlining mechanisms (e.g., plaque rupture, dissection, erosion, eruptive calcific nodules, intraplaque haemorrhage) may require intracoronary imaging.
Permanent or reversible microvascular impairment is a common consequence of ST-segment elevation MI, through microvascular obstruction (MVO) and intramyocardial haemorrhage caused by prolonged ischaemia, inflammation, endothelial dysfunction, oedema, or ischaemia-reperfusion damage. The index of microcirculatory resistance (IMR) is related to the extent of MVO, and a post-percutaneous coronary intervention (PCI) IMR ≥40 is a predictor of poor outcome5.
The reduced vasodilatory capacity and hyperaemic response significantly affect the physiological assessment of the infarct-related artery in the acute phase. At follow-up, the hyperaemic response may increase again and FFR may decrease, with no change in the epicardial stenosis significance, whereas resting indices may be less affected6.
Non-culprit stenosis
Recent evidence in support of physiology-guided revascularisation of intermediate non-culprit lesions in patients presenting with ACS is either positive or neutral. Although its use is supported by randomised clinical trials (RCTs) when compared with culprit-only treatment78, it remains debatable whether a “complete” FFR-guided strategy improves clinical outcomes over angiographic guidance. The deferral of non-flow-limiting, non-culprit stenosis is associated with higher 1-year major adverse cardiac event (MACE) rates than for chronic coronary syndromes (CCS)9. The most recent FLOWER-MI trial failed to show a significant benefit of FFR-guided complete revascularisation over angiographic guidance in terms of death, MI or urgent revascularisation at 1 year10.
The main factors limiting the applicability of physiological guidance in the setting of ACS are listed below and in Figure 1:
- Plaque vulnerability of non-culprit lesions, which increases the risk of later events in spite of non-flow-limiting obstruction11121314.
- Altered haemodynamic and microcirculatory status causing underestimation of functional stenosis significance1516.
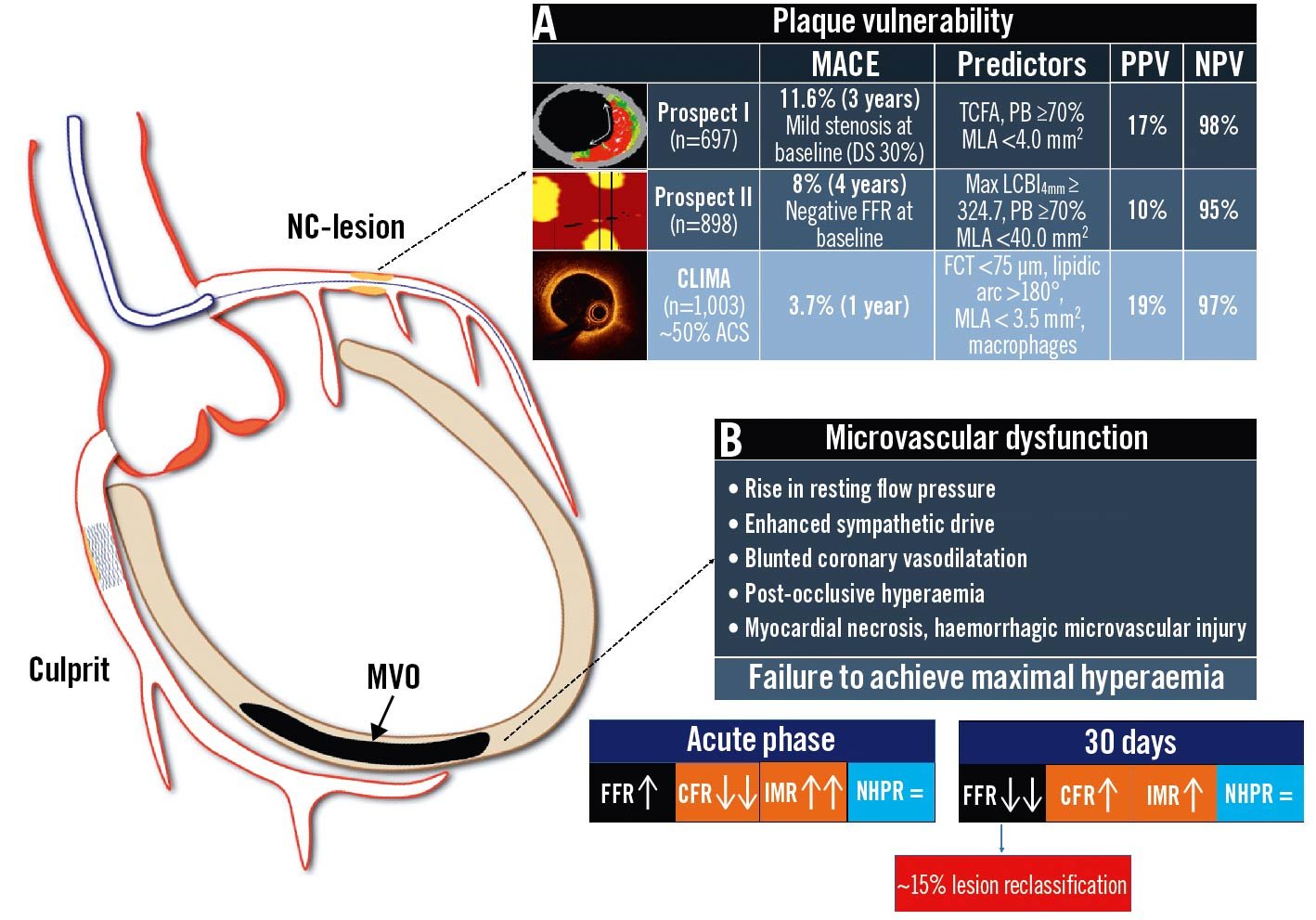
Figure 1. Coronary physiology and non-culprit lesions of acute coronary syndromes: potential pitfalls. Potential scenarios affecting the reliability of invasive physiological assessment of non-culprit lesions in ACS: A. residual non-flow-limiting high-risk vulnerable plaques; B. microvascular impairment and blunted vasodilatory ability leading to reduced hyperaemic coronary flow. ACS: acute coronary syndrome; CCS: chronic coronary syndrome; CFR: coronary flow reserve; DS: diameter stenosis; FCT: fibrous cap thickness; FFR: fractional flow reserve; IMR: index of microcirculatory resistance; LCBI: lipid-core burden index; MACE: major adverse cardiac events; MLA: minimal lumen area; MVO: microvascular obstruction; NC: non-culprit; NHPR: non-hyperaemic pressure ratios; NPV: negative predictive value; PB: plaque burden; PPV: positive predictive value; TCFA: thin-cap fibroatheroma
See ➔ Microvascular dysfunction
Bifurcations
When clinically indicated, there are specific requirements for the functional investigation of a bifurcation lesion to avoid measurement errors. Before intervention, functional testing can be performed on the main vessel (MV) or, in the case of pure SB stenosis (Medina 0,0,1), on the side branch (SB). During provisional stenting across the SB, a jailed SB evaluation should be considered in case of ostial “pinching”, in order to assess the need for additional SB intervention (Figure 2)17. Periprocedural normal FFR or instantaneous wave-free ratio (iFR) values in a jailed SB have been associated with good functional results at follow-up, supporting the adoption of conservative strategies1819. Overall, a jailed SB functional evaluation allows a reduction in the rates of SB stenting during provisional SB treatment20.
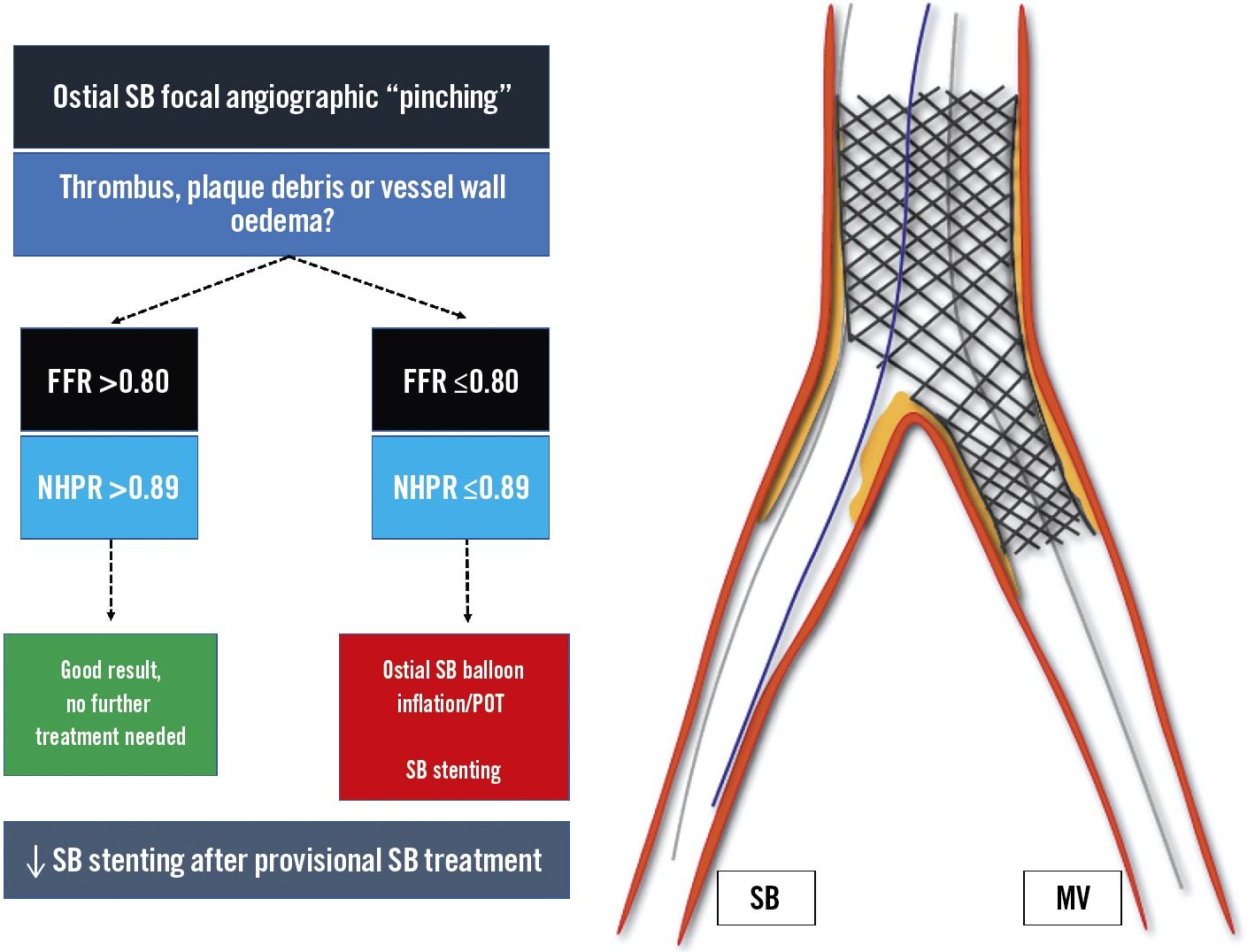
Figure 2. Provisional bifurcation stenting: jailed side branch functional assessment. Following SB provisional stenting the functional evaluation of the jailed side branch should be considered in case of ostial SB “pinching”. In case of FFR >0.80/NHPR >0.89, further SB treatment is not recommended, while in case of abnormal values, ostial SB balloon inflation, POT and eventual SB stenting are suggested. FFR: fractional flow reserve; MV: main vessel; NHPR: non-hyperaemic pressure ratios; POT: proximal optimisation technique; SB: side branch
Recently, the Murray’s law-based quantitative flow ratio (μQFR) has been developed to overcome the impact of linear vessel tapering (Figure 3), previously a limitation of computational QFR in bifurcations stenoses21.
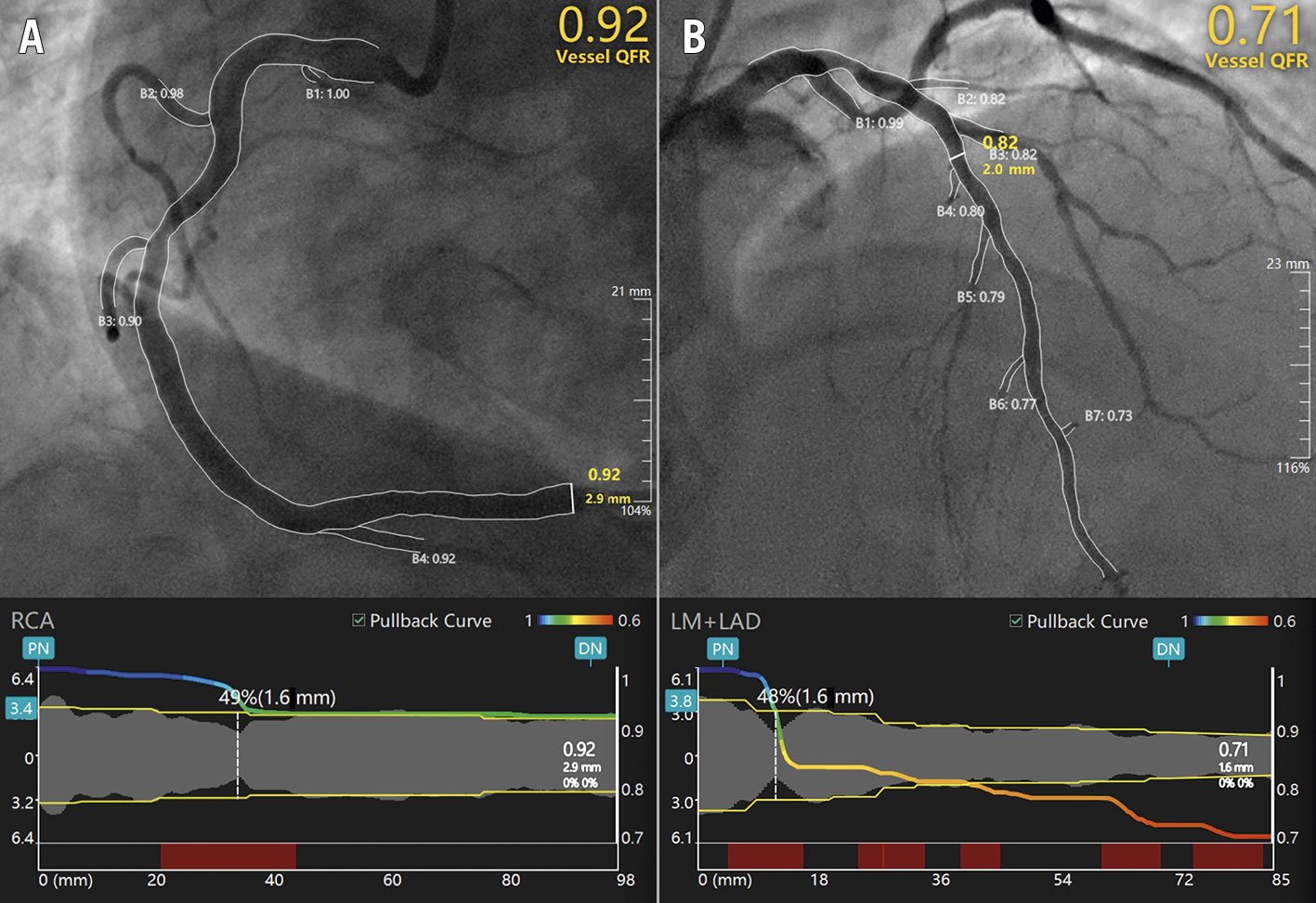
Figure 3. Representative examples of μQFR computation. Upper panels show right (A) and left anterior descending (B) coronary artery disease. The lumen contours and side branches of the target vessels are automatically delineated and superimposed on the angiographic images. A step-down reference diameter function is reconstructed based on Murray’s bifurcation fractal law and used for μQFR computation. The lower panels provide a virtual point-by-point physiologic mapping of the target vessel. DN: distal normal; LAD: left anterior descending artery; LM: left main; PN: proximal normal; QFR: quantitative flow ratio; μQFR: Murray’s law-based QFR; RCA: right coronary artery.
See ➔ Left Main, QFR
CABG
Coronary artery bypass grafting (CABG) is an invasive procedure that supplies extra blood flow to the heart in patients with extensive CAD via surgical implantation of additional conduits.
Whilst observational studies have shown a significant correlation between preoperative FFR and graft patency at follow-up, RCTs have failed to confirm such findings2223. The FARGO and the GRAFFITI RCTs, comparing FFR- and angio-guided surgical strategies, failed to demonstrate improved 6-12 month graft patency nor advantages in terms of clinical events22. In GRAFFITI, FFR-guidance allowed a simplification of the surgical intervention in up to 50% of patients, by reducing the number of anastomoses and by increasing off-pump grafting and minimally invasive surgery2224.
The role of physiological evaluation after CABG is limited. The early post-operative resistance and hyperaemic pressure drop of the left internal mammary appear significantly higher than that of the right or the saphenous vein grafts25. When both native and grafted vessels are patent but diseased, the PW sensor should be placed distally to the anastomosis (either through a native or bypass conduit). With FFR <0.75, further revascularisation is needed, preferably on the native vessel. With FFR >0.80, no invasive treatment is needed2627. Image-based computational functional models have not been fully validated in CABG patients.
See ➔ Multivessel disease, Trials (FAME 3)
Chronic coronary syndromes
In CCS, coronary flow reserve (CFR) impacts on the patient’s exertional tolerance and symptoms of angina or dyspnoea. Several RCTs have outlined how coronary flow restoration and myocardial revascularisation improve anginal symptoms more effectively than optimal medical therapy (OMT)28. However, the ORBITA trial raised doubts about the mechanisms of symptomatic improvement, illustrating the significant role of a placebo effect29. In the large ISCHEMIA trial, a clinically relevant improvement in symptoms at 3-year follow-up was demonstrated, a time point when the placebo effect should be exhausted30. Even in ORBITA, PCI patients with abnormal invasive physiology experienced higher freedom from angina at follow-up31. In addition to symptomatic relief, FFR-guided PCI in CCS improves clinical outcomes, especially reducing the risk of spontaneous MI in comparison to OMT alone32. The net benefit of revascularisation depends on the risk balance: at higher FFR values, already low adverse event rates cannot be further improved and deferral appears to be safer33. In the lower range of FFR values, the incidence of MI and cardiac death increases and the benefit of revascularisation becomes apparent, as long as the procedural risk is low34.
See ➔ Guidelines
CT-derived physiology
CT-FFR
Computed tomography-derived FFR (CT-FFR) is based on the integration of a 3-dimensional anatomical reconstruction of the coronary tree and computational fluid dynamics. CT-FFR was developed to overcome the low specificity of coronary computed tomography angiography (CCTA)35. CT-FFR demonstrated a higher positive predictive value (65%) compared to CCTA (40%) in the HeartFlowNXT trial36. In the PACIFIC study37, having FFR as the standard reference, diagnostic metrics were superior to other non-invasive tests (CCTA, myocardial perfusion scintigraphy and positron-emission tomography). CT-FFR allowed for the reallocation of patient management to OMT or revascularisation in 36% of patients in the FFRCT-RIPCORD study38. In a retrospective analysis of the SYNTAX II registry39 and in the prospective SYNTAX III Revolution RCT, CT-FFR proved to be an accurate tool in assessing the stenosis significance in patients with 3-vessel disease, showing an excellent agreement on clinical decisions (which segments needed to be revascularised) and treatment choices (surgery or PCI)40. In the near future, CT-FFR may become the new gatekeeper to invasive interventions, with the aim of reducing costs, radiation exposure and adverse events.
CT-QFR
Computed tomography-derived QFR (CT-QFR) is a novel non-invasive physiology index that applies QFR computational algorithms to CCTA. CT-QFR showed better accuracy than scintigraphy and magnetic resonance41. The advantages of CT-QFR (over CT-FFR) are the fast analysis time and the inclusion of distal vessels down to 1.5 mm42. A case example is provided in Figure 4.
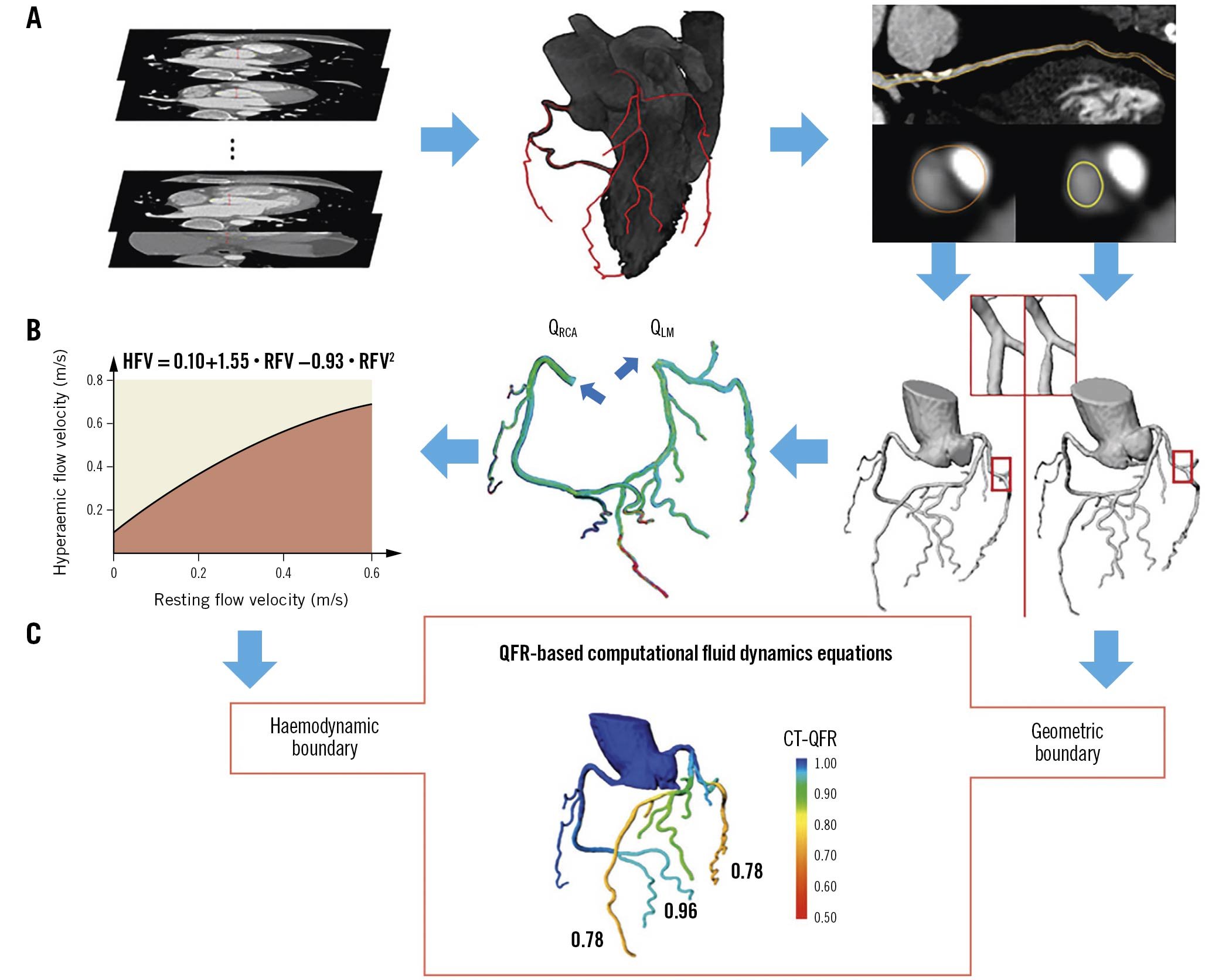
Figure 4. Computed tomography-derived quantitative flow ratio. The framework of CT-QFR computation includes: A) lumen contour and coronary tree automatic reconstruction and CT-QFR computation; B) patient-specific virtual hyperaemic flow estimation; C) QFR computational fluid dynamics equations. CT: computed tomography; HFV: hyperaemic flow velocity; LM: left main; QFR: quantitative flow ratio; RCA: right coronary artery; RFV: resting flow velocity. Adapted with permission from Li et al42.
See ➔ QFR
Discordances
FFR and non-hyperaemic pressure ratios (NHPR) disagree in defining the physiological significance of coronary stenoses in up to ~20% of cases4344. Several factors have been associated with FFR/NHPR discordances, including lesion location – more often in the left anterior descending (LAD) than other vessels – age, multivessel disease, non-ST-segment elevation MI, smoking and hypertension44. The physiological phenotypic pattern of epicardial CAD seems to have a crucial impact on FFR/NHPR discordances (Figure 5). Diffuse disease predominantly causes friction losses and abnormal NHPR (with preserved FFR) while focal disease predominantly causes separation losses and abnormal FFR (with preserved NHPR)4345. Although no clinical outcome–related data provide a definition for the optimal treatment strategy in case of FFR/NHPR discordance, PW-pullback may offer a potential solution by offering individualised management targeted on the physiological disease phenotype.
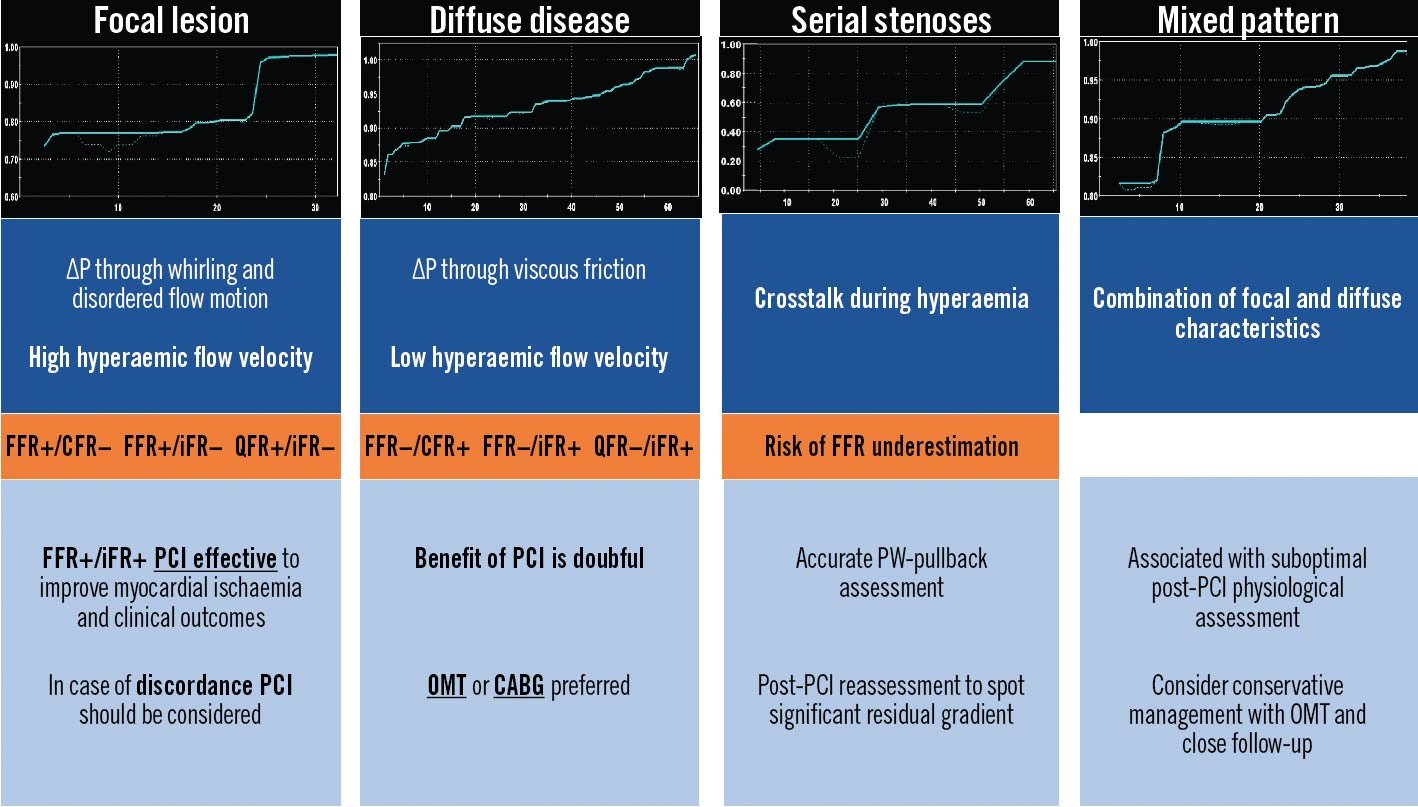
Figure 5. Physiological patterns of epicardial atherosclerosis: pitfalls and clinical relevance. PW-based and angio-derived indices allow the reconstruction of a point-by-point physiological map along the diseased coronary artery. The physiological pattern is relevant for the appropriate CAD management. CABG: coronary artery bypass grafting; CFR: coronary flow reserve; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PW: pressure wire; QFR: quantitative flow ratio; ΔP: change in pressure
See ➔ Grey zone, Patterns of epicardial atherosclerosis
Fractional flow reserve
FFR expresses the maximal achievable blood flow to a myocardial territory in the presence of a stenosis, in the form of a ratio to the maximum achievable flow in the absence of stenosis. FFR is calculated as the ratio of 2 pressures. During maximal hyperaemia, coronary flow and coronary pressure (Pd/Pa) achieve a linear correlation, as coronary resistance is kept stable and minimal. By translating coronary flow into a pressure ratio, FFR becomes independent of haemodynamic conditions46. However, FFR is influenced by the size of the downstream myocardial flow distribution volume and by the presence of collaterals47. Indeed, the larger the amount of “viable” myocardial mass perfused, the more the hyperaemic flow increases and, thus, the higher the pressure gradient (the lower the FFR) for any given stenosis (Figure 6).
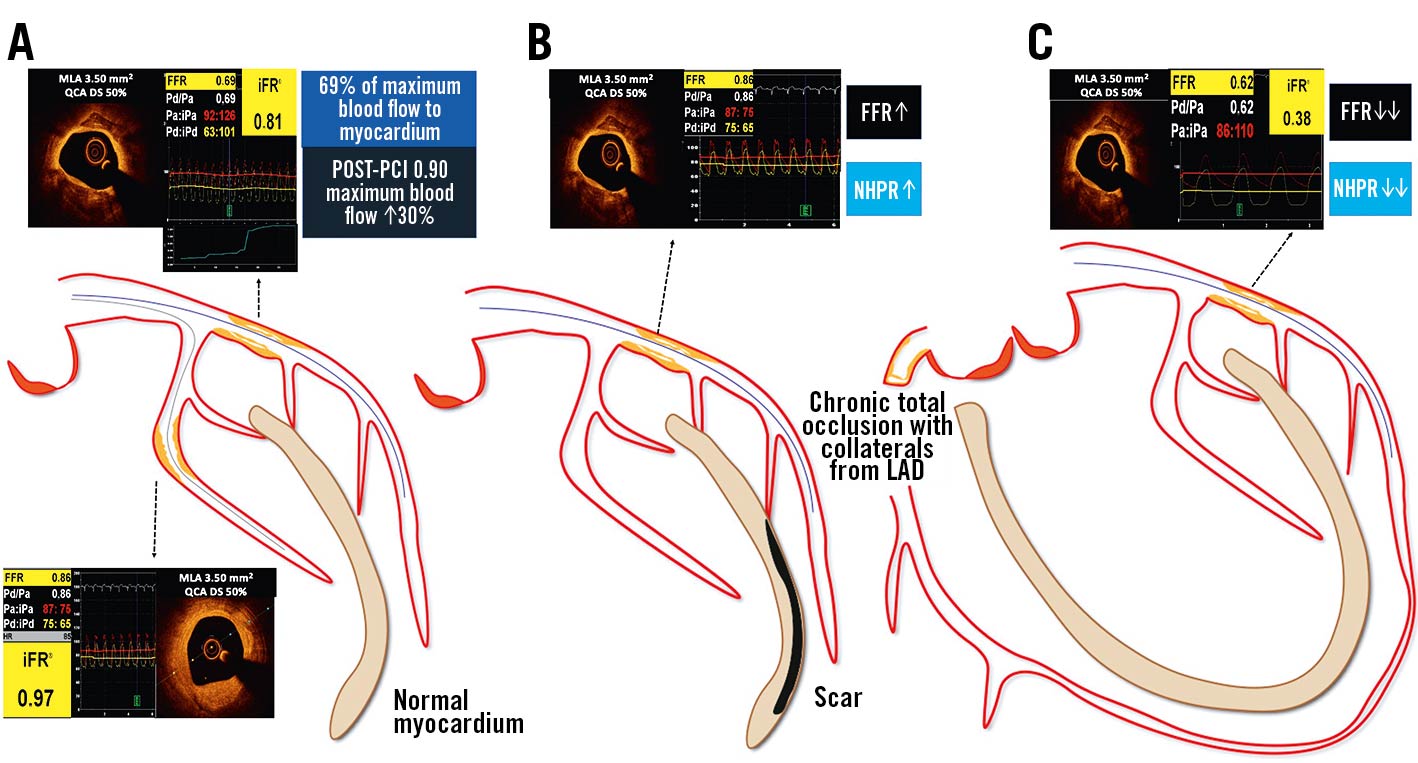
Figure 6. Impact of the amount of supplied myocardial mass on invasive physiology assessment. The larger the amount of “viable” myocardial mass perfused, the higher the pressure gradient for any given stenosis: A) in case of normal myocardium, an epicardial stenosis on the proximal LAD has a different haemodynamic relevance compared to the second marginal branch, despite superimposable angiographic DS and OCT derived MLA; B) after a myocardial infarction with necrosis the amount of viable myocardial mass is reduced and the same stenosis yields a lower haemodynamic relevance; C) conversely, in the case of a collateral-donor vessel, the amount of subtended myocardial mass is larger and the pressure gradient higher. DS: diameter stenosis; FFR: fractional flow reserve; iPa: instantaneous aortic pressure; iPd: instantaneous distal pressure; LAD: left anterior descending; MLA: minimal lumen area; NHPR: non-hyperaemic pressure ratios; OCT: optical coherence tomography; Pa: aortic pressure; Pd: distal pressure; PCI: percutaneous coronary intervention; QCA: quantitative coronary angiography
See ➔ Grey zone, Normal values, Tips and Tricks, Trials (FAME)
Grey zone
FFR-validated ischaemic (<0.75; revascularisation justified) and non-ischaemic (>0.80; PCI deferral) thresholds allow stenosis-specific clinical decision-making. FFR values between 0.76 and 0.81, seen in fewer than 10% of cases, are sometimes considered the “grey zone” with an uncertain diagnostic value. Different cut-off values inside the grey zone have been proposed for different subsets (i.e., left main, diabetes, MI, multivessel disease, heart failure) but, to date, consensus is lacking47. Only observational studies have compared deferral and revascularisation in “grey-zone” cases. The net clinical benefit of revascularisation over medical treatment increases as the FFR values decrease, with a sharp continuum of risk below the 0.80 threshold3448. In borderline cases, individualised care based on good clinical judgement should consider symptoms, results of non-invasive tests when available, adherence to OMT, procedural risks as well as the physiological pattern of CAD47.
See ➔ Chronic coronary syndromes, Patterns of epicardial atherosclerosis
Guidelines
According to the National Institute for Health and Care Excellence guidelines, patients with CCS should be managed medically by default. In case of uncontrolled symptoms, ICA to guide further treatment strategy should be considered, while additional invasive functional testing may be required for evaluation of angiographic findings and tailored treatment decisions. In patients with CCS and FFR <0.80, outcomes are better for FFR-guided PCI than for OMT alone49.
European and American guidelines state that in case of angina or angina equivalent, without documented ischaemia and angiographically intermediate stenoses, the use of FFR or iFR is recommended for risk stratification and to proceed with revascularisation2850.
Several studies have underlined the cost-effectiveness of FFR measurement in the cath lab, showing that FFR-guided PCI compared to angiography portends risk reduction and improved health, while cost is reduced51. The technology qualifies as “disruptive”: better outcomes at lower cost.
The adoption of physiological guidance for revascularisation in clinical practice is heterogeneous and globally low (~20%), due to technical and economic issues, as well as to resistance to change, explaining why decisions remain largely driven by angiography alone 52. New PW- and adenosine-free (NHPR) alternatives to FFR were expected to increase the adoption of invasive physiology in a real-world decision-making process. However, according to the ISIS 2 survey, the perceived need for invasive physiology remains low, even when facing stenoses of intermediate angiographic severity. As a result, decisions have remained based purely on angiography alone in more than 60% of cases. Of these, decisions were discordant with functional assessment in up to 40% of the cases, mainly resulting in overtreatment (i.e., PCI in spite of preserved FFR)53.
See ➔ Chronic coronary syndromes, Trials
Haemodynamic variables
Haemodynamic conditions of the patient are prone to variation during interventional procedures, with sudden changes in blood pressure and heart rate. The simultaneous measurement of aortic and distal pressures, as well as the ability of the microcirculation to vasodilate to the same extent, allows FFR measurements to be reproducible over a wide range of conditions4754. However, at heart rates >110 bpm, FFR may be underestimated55. The NHPR seems to be influenced by haemodynamic changes in a similar way56.
Systemic conditions that increase resting cardiac output and/or coronary flow (i.e., anaemia, sepsis, hyperthyroidism, myeloproliferative disorders, arteriovenous fistula, chronic kidney disease, liver cirrhosis, and Paget’s disease) may decrease NHPR values, without affecting hyperaemia, and consequently have no significant effect on FFR5758.
Increased left ventricular end-diastolic pressure reflected in increased central venous pressures is linearly related to FFR/NHPR values. Coronary microvascular dysfunction (CMD) and blunted vasodilatory ability during hyperaemia will result in a falsely increased FFR. Increased resting end-diastolic pressure may result in underestimated NHPR values59. In daily practice, the clinical relevance of these observations seems negligible, with less than 10% reclassification60. A case example is provided in Figure 7.
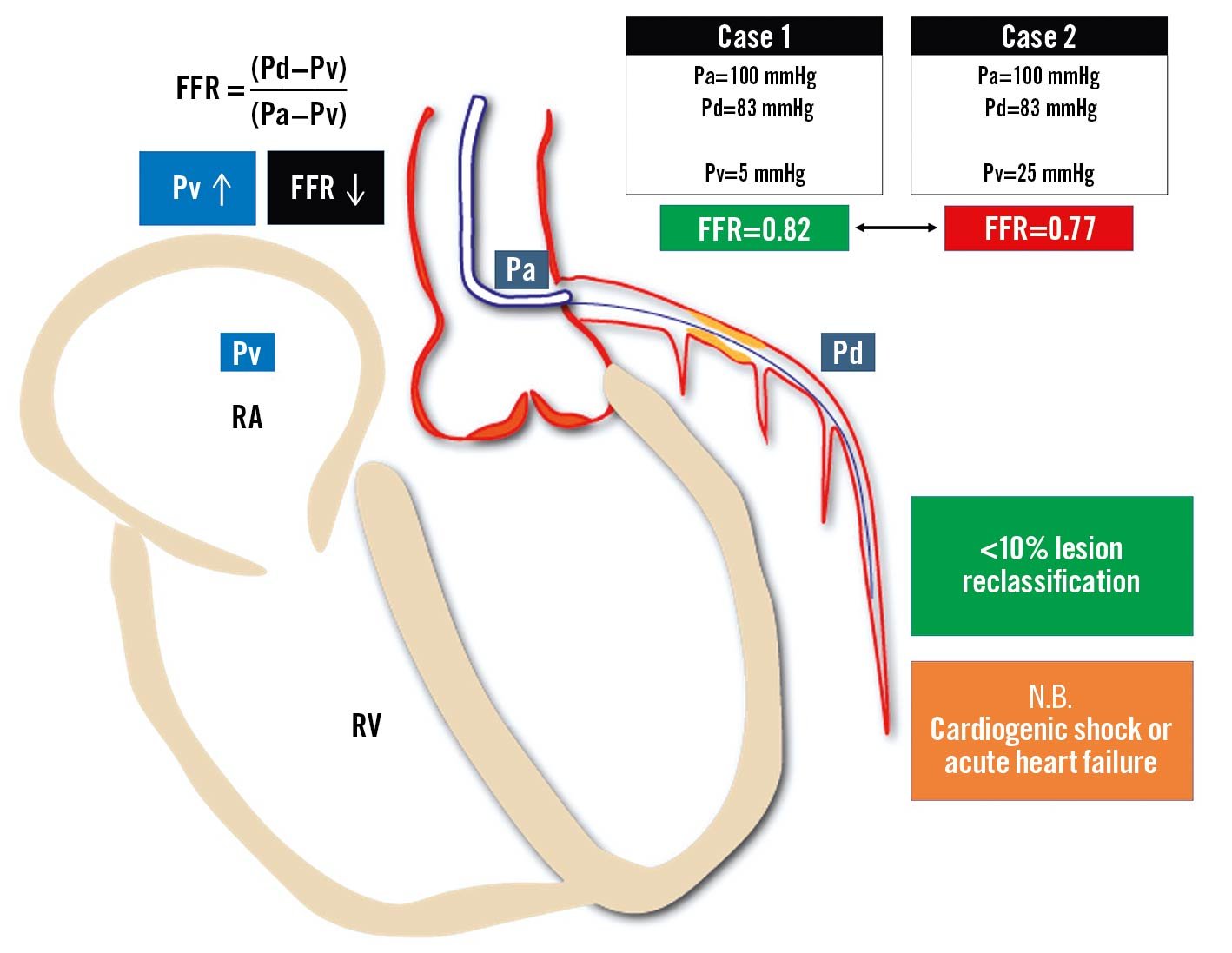
Figure 7. Influence of central venous pressure on fractional flow reserve measurement. An increase of central venous pressure is related to a progressive reduction of the FFR value: the same Pd and Pa values measured across an epicardial stenosis provide a negative FFR value in cases with normal Pv (Case 1) or a positive FFR value in cases with elevated Pv (Case 2). Even though normal- to moderately-increased Pv does not affect FFR in a clinically relevant way, FFR calculated without accounting for severely increased Pv can be significantly overestimated in case of acute heart failure or cardiogenic shock. FFR: fractional flow reserve; Pa: aortic pressure; Pd: distal pressure; Pv: venous pressure; RA: right atrium; RV: right ventricle
See ➔ Microvascular dysfunction
Hyperaemia
Hyperaemia is the mechanism that allows blood flow to meet the metabolic needs of the heart. Pharmacologically-induced stable maximal hyperaemia represents the cornerstone of FFR because minimal coronary resistance results in a linear relation between coronary flow and pressure. After relief of potential vasoconstriction by intracoronary nitrates (200 µg), several hyperaemic agents can be used (Figure 8). Adenosine, administered intravenously (IV) or intracoronarily (IC), is most commonly used47. IV adenosine can be administered for minutes in a central vein, allowing pullback manoeuvres in stable hyperaemic conditions, and is preferred in case of serial or diffuse disease and in case of FFR/NHPR discordance46. In case of focal disease, IC adenosine can provide similar FFR values to IV administration61. Regadenoson and papaverine are valid alternatives to adenosine, providing a prolonged hyperaemic effect (approximately 2-5 min and 1 min, respectively)626364.
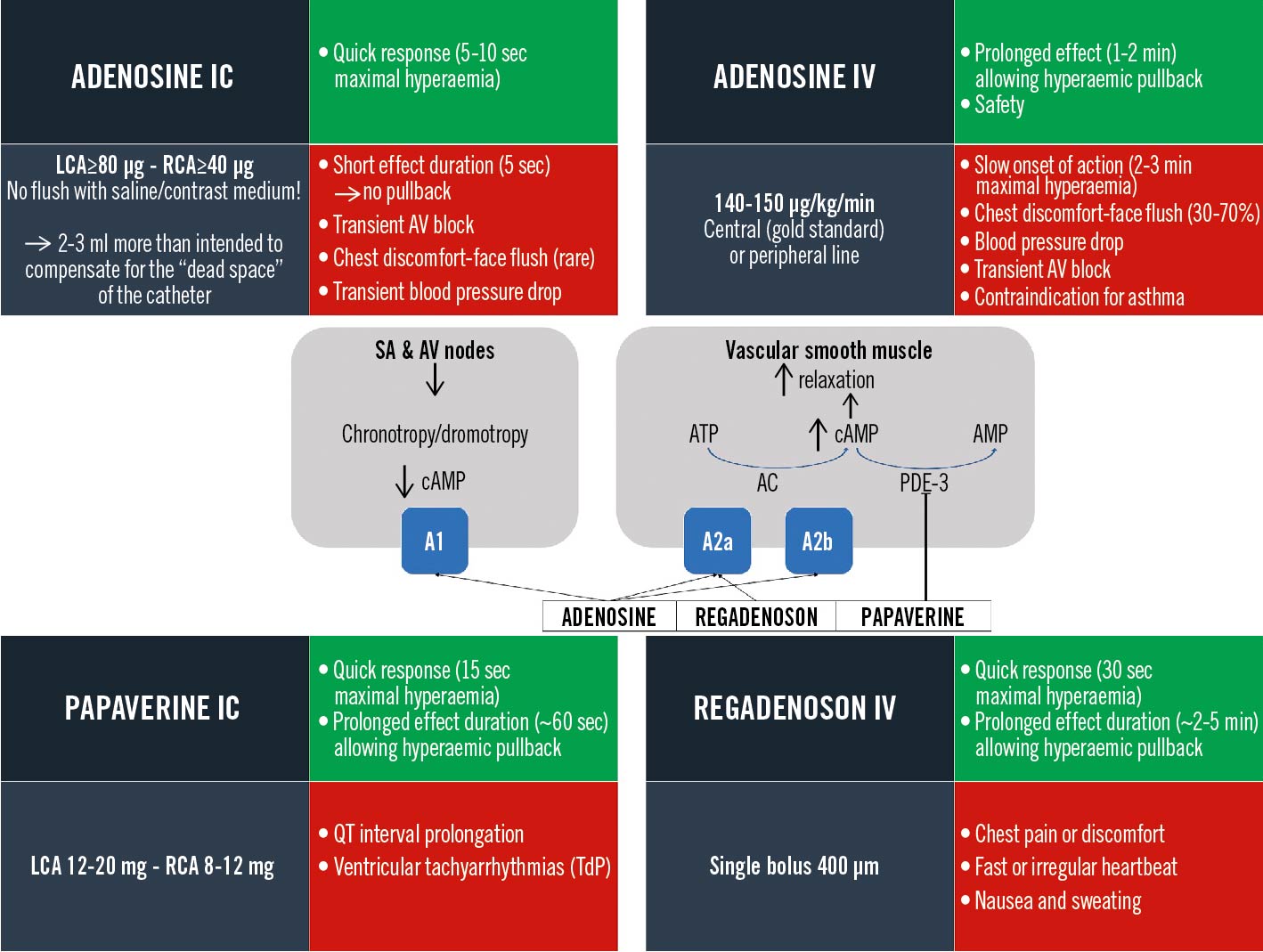
Figure 8. Hyperaemic agents compendium: mechanism of action and user manual. Posology (grey panels), advantages (green panels) and pitfalls/side effects (red panels). A1-A2: adenosine receptors; AC: adenylyl cyclase; AMP: adenosine monophosphate; ATP: adenosine triphosphate; AV: atrioventricular; cAMP: cyclic adenosine monophosphate; IC: intracoronary; IV: intravenous; LCA: left coronary artery; PDE-3: phosphodiesterase-3; RCA: right coronary artery; SA: sinoatrial
See ➔ FFR, Tips and Tricks
iFR and NHPR
The NHPR family comprises several indices (Figure 9) that provide physiological assessments in baseline conditions in a cheaper and faster way and with fewer side effects than FFR. All of these indices report Pd/Pa ratios that differ depending on the phase of the cardiac cycle during which Pd/Pa ratios are measured. Outcome data are only available for FFR and iFR, while other NHPR indices were validated against FFR and/or iFR.
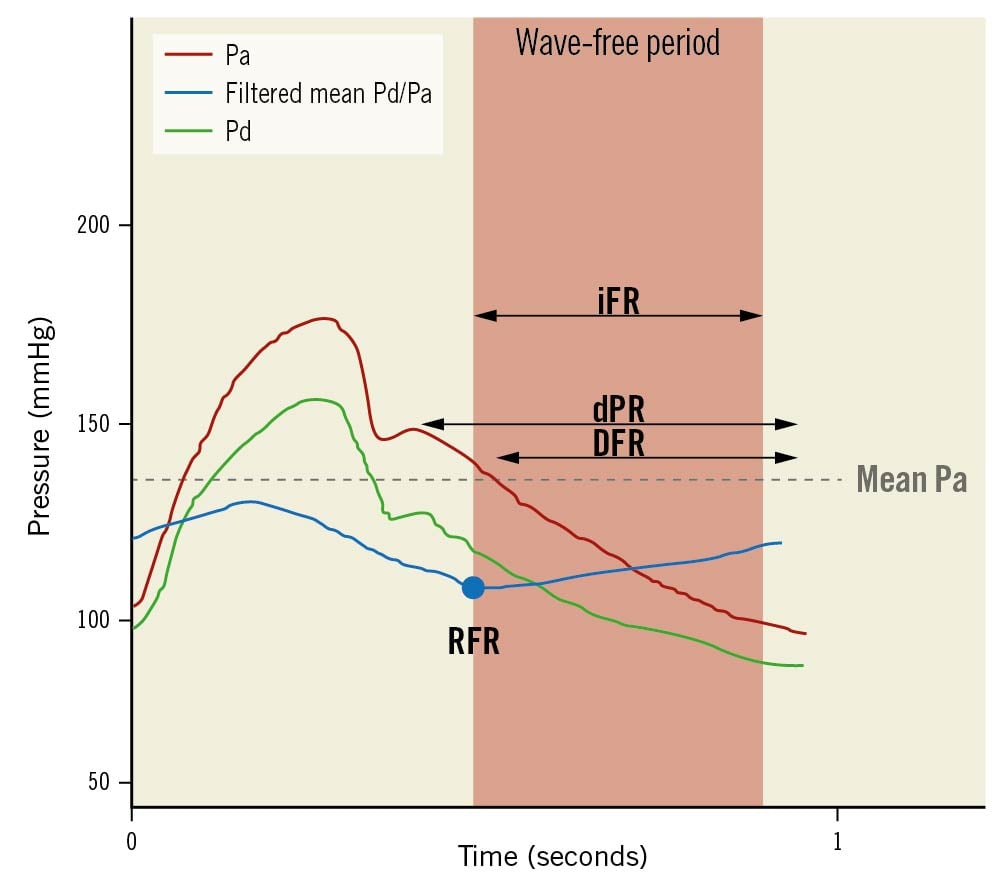
Figure 9. Non-hyperaemic pressure ratios. iFR is the average Pd/Pa measured in the wave-free period. dPR is the average Pd/Pa along the whole diastole. DFR is defined as average Pd/Pa measured in the phase with Pa lower than mean Pa. RFR is the lowest mean Pd/Pa detected inside the whole cardiac cycle. DFR: diastolic hyperaemia-free ratio; dPR: diastolic pressure ratio; iFR: instantaneous wave-free ratio; Pa: aortic pressure; Pd: distal pressure; RFR: resting full-cycle ratio. Adapted with permissions from Kogame et al66.
The instantaneous wave-free ratio (iFR) picks up Pd/Pa values during a particular diastolic phase (wave-free period), when resistance is relatively constant. Yet, iFR is sensitive to noise, wire drift and variations in haemodynamic conditions that may modify resting coronary flow, including contrast injections6566.
Contrast FFR is the lowest mean Pd/Pa after contrast medium injection and, having FFR as a reference, has shown better accuracy than iFR or Pd/Pa67. The resting full-cycle ratio reflects the lowest instantaneous Pd/Pa within the entire cardiac cycle, requiring at least 5 consecutive beats for proper acquisition, and allows both online and off-line measurement, with results superimposable to iFR (97.4% accuracy). Interestingly, resting the full-cycle ratio was measured in ~10% of cases outside diastole (>30% in the right coronary artery)68.
See ➔ Normal values, Tips and Tricks, Trials (DEFINE-FLAIR and IFR-SWEDEHEART)
Imaging
Unlike ICA, intracoronary imaging techniques allow a precise evaluation of the cross-sectional morphology of the arterial vessel wall, thereby providing insightful support for PCI planning and optimisation. To date, the integrated approach of either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) with wire-based physiology is often limited due to additional costs and procedural time. Novel models have been developed allowing the physiology to be directly derived from intravascular imaging acquisition through the fusion with 3-dimensional angiography and application of computational fluid dynamics. ICA remains the GPS of these acquisitions through simultaneous registration and point-by-point vessel reconstruction69.
To date, head-to-head comparisons between functional and intracoronary imaging-guided revascularisation have been sparce. In the hypothesis-generating single-centre FORZA trial70, OCT guidance was associated with a lower incidence of the composite of MACE or significant angina at 13 months, whereas in the large FLAVOUR trial, IVUS- and FFR-guided PCI were associated with a similar incidence of adverse cardiovascular events at 24 months71.
See ➔ OFR, QFR, UFR, Virtual PCI
Intermediate stenoses
Intermediate stenoses are those with an angiographically defined DS of 50-90% and unpredictable haemodynamic significance. Invasive physiology can identify those lesions that are haemodynamically significant, i.e., reducing maximum achievable flow and exercise tolerance. FFR is considered the gold standard for the assessment of functional significance of intermediate lesions, with a greater accuracy than exercise electrocardiography, myocardial perfusion scintigraphy and stress echocardiography. In patients with CCS, angio-guided PCI only provides appropriate management in ~50% of cases compared to FFR, with the other ~30% having inappropriate PCI and ~20% having inappropriate deferral72. Conservative management of lesions with preserved FFR is favourable, with an estimated ~1% risk of MI and cardiac death per year that cannot be further improved by PCI. Conversely, an ischaemic lesion has a ~5% per year risk of events, which can be reduced by revascularisation4773.
See ➔ Chronic coronary syndromes, Guidelines
Left main
Left main (LM) is the largest bifurcation vessel in the coronary tree, supplying more than 50% of the total myocardial mass and often presenting the left circumflex (LCx) artery as an SB. A significant LM stenosis is associated with poor outcome. Its accurate evaluation by angiography is difficult, and non-invasive assessment is often misleading due to “balanced ischaemia” and falsely normal results47. Invasive physiological assessment of LM disease with FFR or NHPR has been widely validated, providing evidence that deferred treatment according to FFR >0.80 or NHPR >0.89 is safe7475. However, LM disease is rarely isolated, and often associated with concomitant LAD and/or LCx stenoses. Therefore, both the LAD and LCx should be assessed, including a PW-pullback manoeuvre, in order to evaluate the impact of LAD/LCx disease on the LM FFR value17. Particular attention should be paid to the correct equalisation of proximal and distal pressures, to be obtained after disengaging the guiding catheter. Moreover, hyperaemia should be induced preferentially by IV adenosine47. A comprehensive illustration of the procedure is provided in Figure 10.
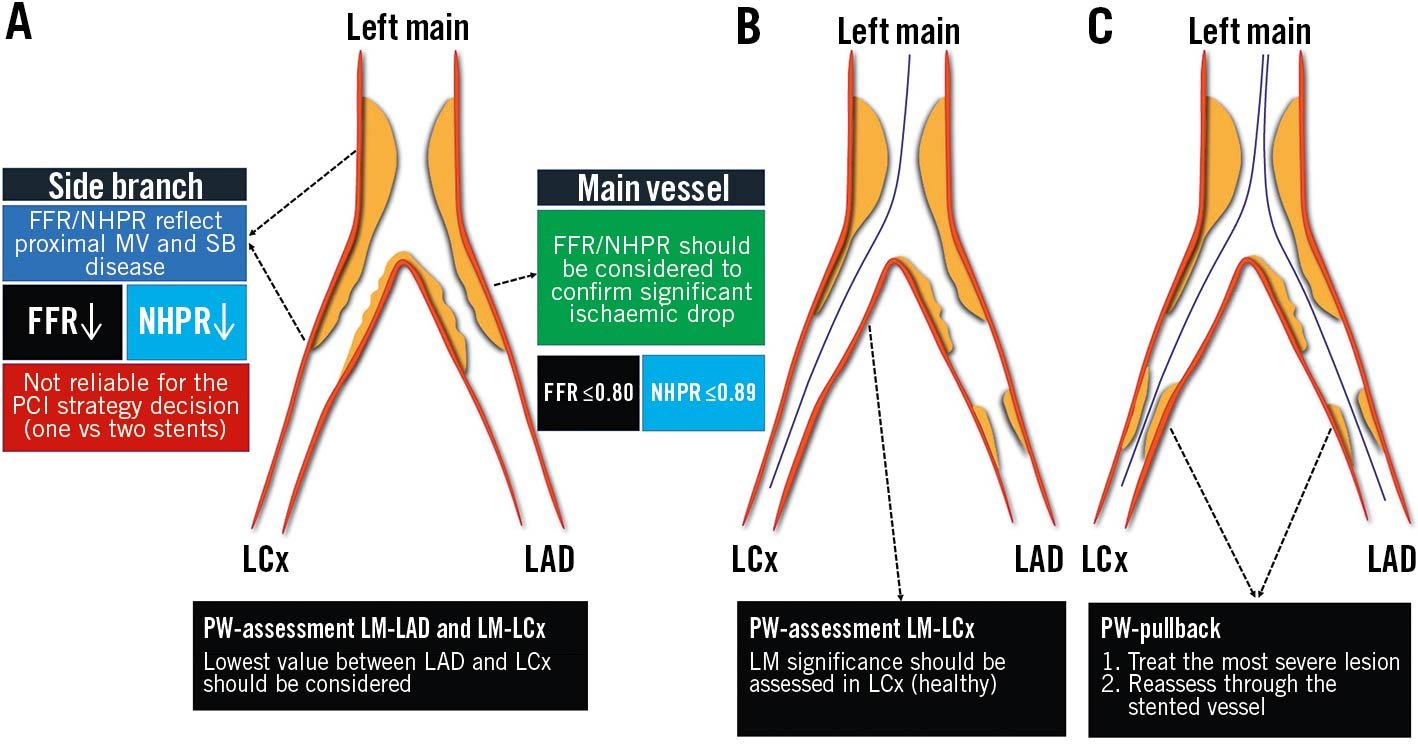
Figure 10. Tips and tricks for invasive physiology in left main bifurcation. Three scenarios are presented: isolated LM bifurcation disease (A); concomitant LM bifurcation and LAD disease (B); concomitant LM bifurcation, LAD and LCx disease (C). FFR: fractional flow reserve; LAD: left anterior descending; LCx: left circumflex; LM: left main; MV: main vessel; NHPR: non-hyperaemic pressure ratio; PCI: percutaneous coronary intervention; PW: pressure wire; SB: side branch
See ➔ Bifurcations
Microvascular dysfunction
CMD is a common condition that causes or worsens ischaemia in patients with or without obstructive epicardial CAD. The combined measurement of FFR and CFR accounts for the evaluation of CMD with frequent (up to 40%) cases of disagreement. Whereas the management of FFR abnormal/CFR abnormal (revascularisation), FFR normal/CFR normal and FFR normal/CFR abnormal (medical therapy) has been established, the prognostic relevance and the best management of patients with discordant FFR ≤0.80 (abnormal) and CFR ≥2.0 (normal) remains undefined. It was suggested that preserved CFR may avoid vessel failure and allow deferral of PCI, but this strategy has provided suboptimal results in the DEFINE-FLOW study76. Direct evaluation of CMD by the index of microcirculatory resistance (IMR) might allow improved risk stratification and inform targeted medical therapy with improved symptomatic control77.
Of note, CMD affects FFR measurements through blunted hyperaemic flow response, leading to an underestimation of stenosis significance in conditions such as ACS and aortic stenosis.
See ➔ Acute coronary syndromes, TAVI, Thermodilution, Transplant vasculopathy
Multivessel disease
The evidence in support of FFR-guidance in the setting of multivessel disease is still a matter of debate, whilst CABG remains the treatment of choice for 3-vessel CAD with moderate-to-severe anatomic complexity, high disease burden and in the presence of diabetes787980. FFR-guided PCI with new-generation drug-eluting stents (DES) promised far better outcomes with fewer stent implants818283. The FAME 3 trial was conceived against this background but failed to prove non-inferiority of FFR-guided PCI compared to CABG guided by ICA. The FUTURE trial even failed to show the benefit of FFR over standard ICA as the gatekeeper for revascularisation decisions (CABG and PCI) in multivessel CAD84. Several trial limitations should be acknowledged, such as premature study termination due to safety issues (not confirmed at the 12-month analysis) and the inclusion of a heterogeneous patient population (up to 50% with ACS, high SYNTAX score)84. A comprehensive illustration is provided in Figure 11.
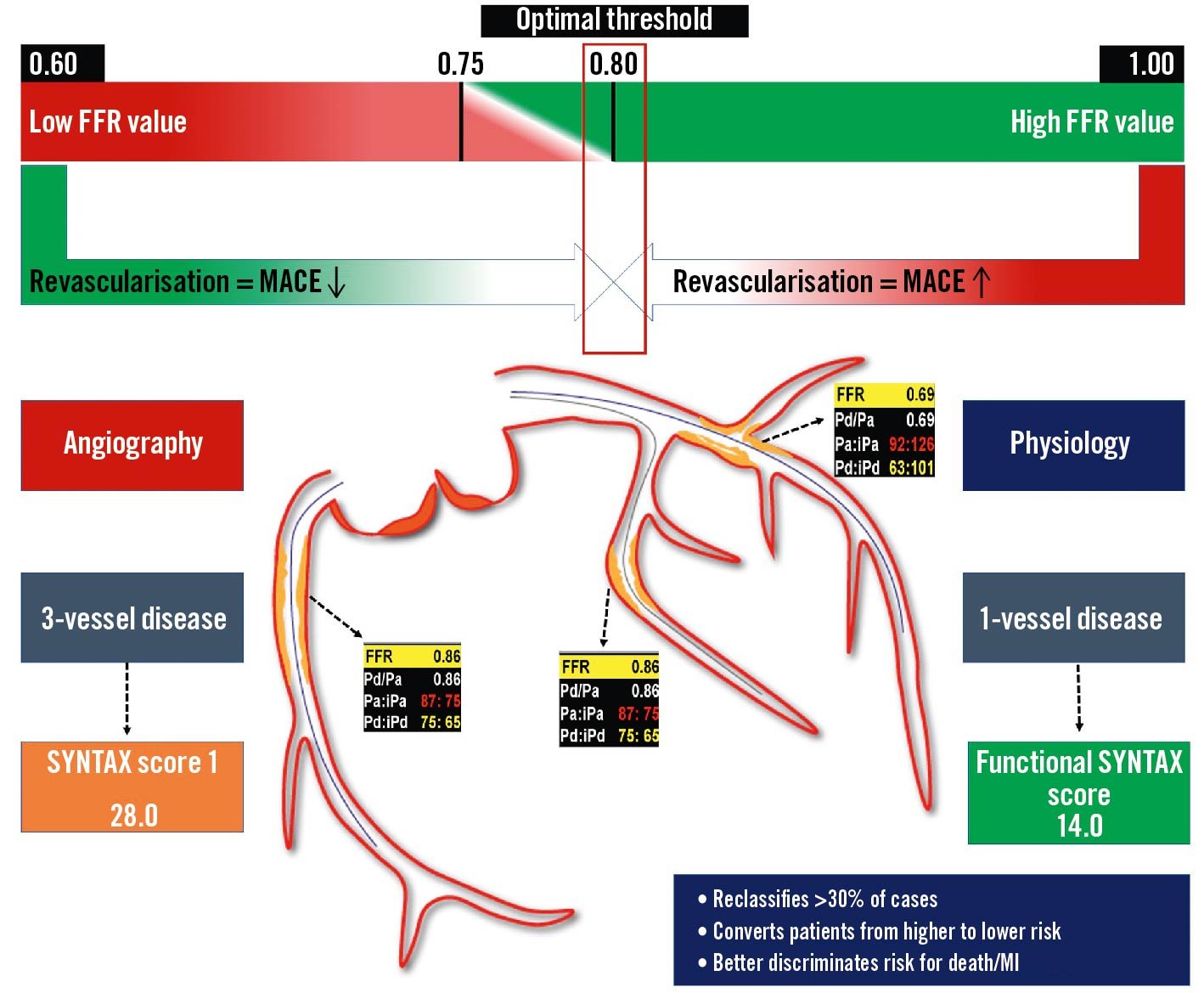
Figure 11. Invasive physiology for the guidance of multivessel disease revascularisation. The use of invasive physiology in case of multivessel disease allows a reclassification of lesion severity, reducing the number of haemodynamically significant lesions. A patient with angiographic 3-vessel disease actually presents a haemodynamically significant single-vessel disease (LAD) at the FFR evaluation, reducing the SYNTAX score from 28 to 14. FFR: fractional flow reserve; iPa: instantaneous aortic pressure; iPd: instantaneous distal pressure; LAD: left anterior descending; MACE: major adverse cardiac events; MI: myocardial infarction; Pa: aortic pressure; Pd: distal pressure
See ➔ Trials (FAME)
Normal range and values
Completely normal epicardial coronary arteries show no decrease in pressure and flow during maximal hyperaemia and the “normal” FFR is 1.085. Progressive obstacles to maximal coronary flow and pressure increase with plaque burden and, therefore, reduced epicardial coronary conductance show a continuum with advanced age. As such, a dichotomic assessment of FFR and NHPR appears trivial. FFR showed high accuracy in detecting haemodynamically significant stenoses, and FFR <0.75 predicts inducible ischaemia with 100% specificity, whereas FFR >0.80 excludes it with a specificity of 90%8687. FFR values between 0.76 and 0.81, seen in less than 10% of cases, are sometimes considered as a “grey zone” with an uncertain diagnostic value. The NHPR-validated cut-off for significance is ≤0.89, while the ≤0.80 threshold is recommended for angio-based and other imaging-derived FFR indexes. Contrast FFR has a cut-off for significance at 0.83, while values above 0.88 relate to a safe deferral. The best target for optimal post-PCI FFR/iFR has not been prospectively determined. A comprehensive illustration is provided in Figure 12.
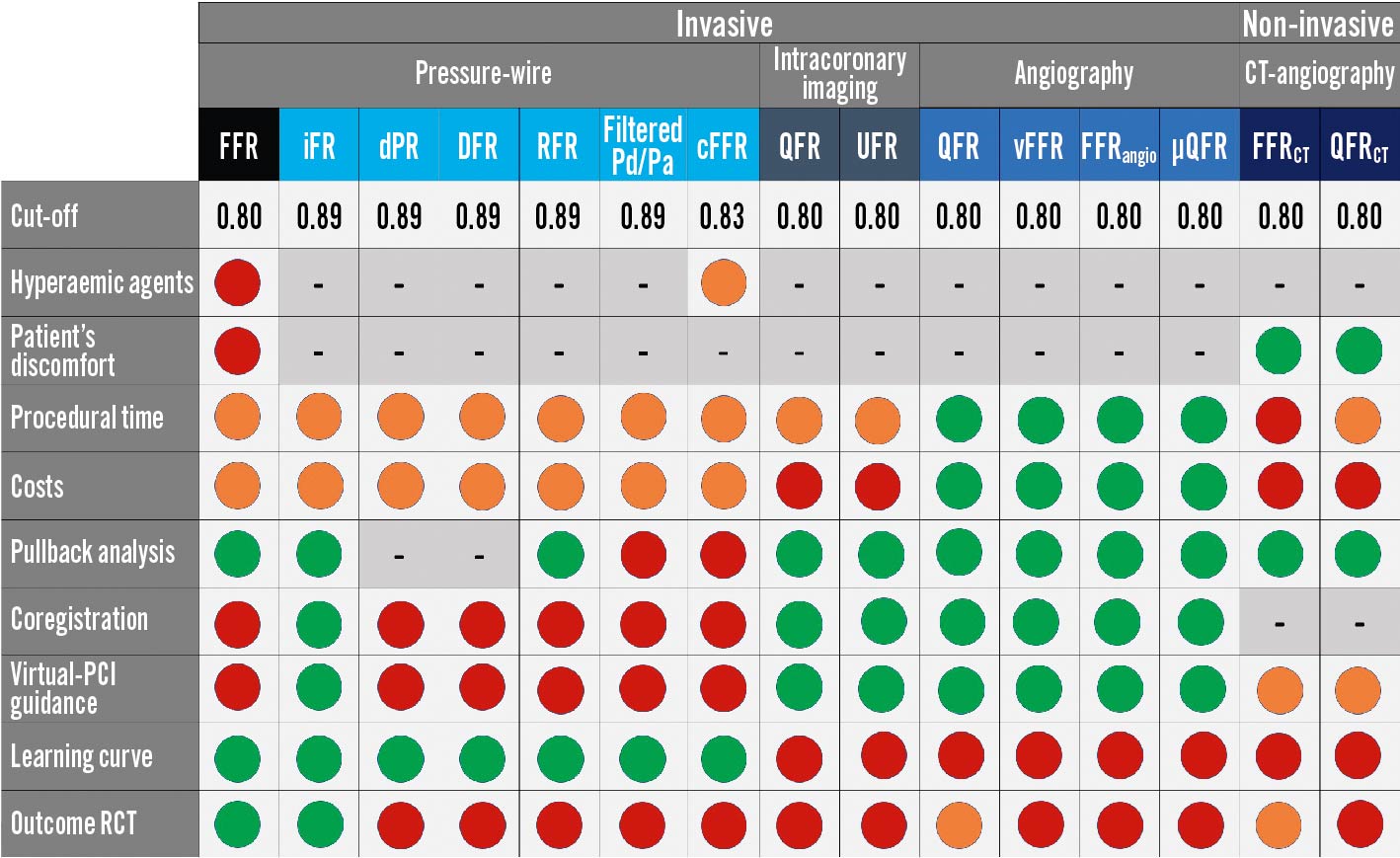
Figure 12. Coronary physiology in the catheterisation laboratory, the complete “arsenal”: pros and cons. The different physiology indices are classified according to a traffic light colour-code, from strength (green) to weakness (red) or intermediate grading (orange). µQFR: Murray’s law-based quantitative flow ratio; CCTA: coronary computed tomography angiography; cFFR: contrast FFR; DFR: diastolic hyperaemia-free ratio; DPR: diastolic pressure ratio; FFR: fractional flow reserve; FFRangio: fractional flow reserve angio; FFRCT: computed tomography–derived FFR; iFR: instantaneous wave-free ratio; OFR: optical flow ratio; PCI: percutaneous coronary intervention; Pd/Pa: cresting distal coronary pressure to aortic pressure ratio; QFR: quantitative flow ratio; QFRCT: computed tomography-derived QFR; RCT: randomised clinical trials; RFR: resting full-cycle ratio; UFR: ultrasonic flow ratio; vFFR: vessel FFR
See ➔ Grey zone
OFR
Optical flow ratio (OFR; OctPlus, Pulse Medical Technology Inc.) is a computational index that integrates OCT and angio-derived physiology, with no need for a dedicated PW or hyperaemia. Lumen contours and 3-dimensional reconstruction are automatically delineated from the OCT image pullback and computational fluid dynamics are applied. The fractal law is used to correct the natural change in lumen size due to the step-down phenomenon allowing a reliable assessment in case of bifurcations. OFR has been validated against invasive wire-based FFR in de novo lesions and in-stent restenoses, showing high diagnostic accuracy (>90%), with negligible intra- and inter-observer variability and low computation time (55±23 seconds)8889. In the setting of virtual PCI and augmented reality for procedural planning, simulated residual OFR and post-PCI OFR showed good accuracy (>84%) in predicting optimal post-PCI FFR ≤0.9090. To date, a single OCT catheter provides appealing morpho-functional information for PCI planning, guidance and optimisation, especially in cases of complex, diffuse or serial stenoses (Figure 13).
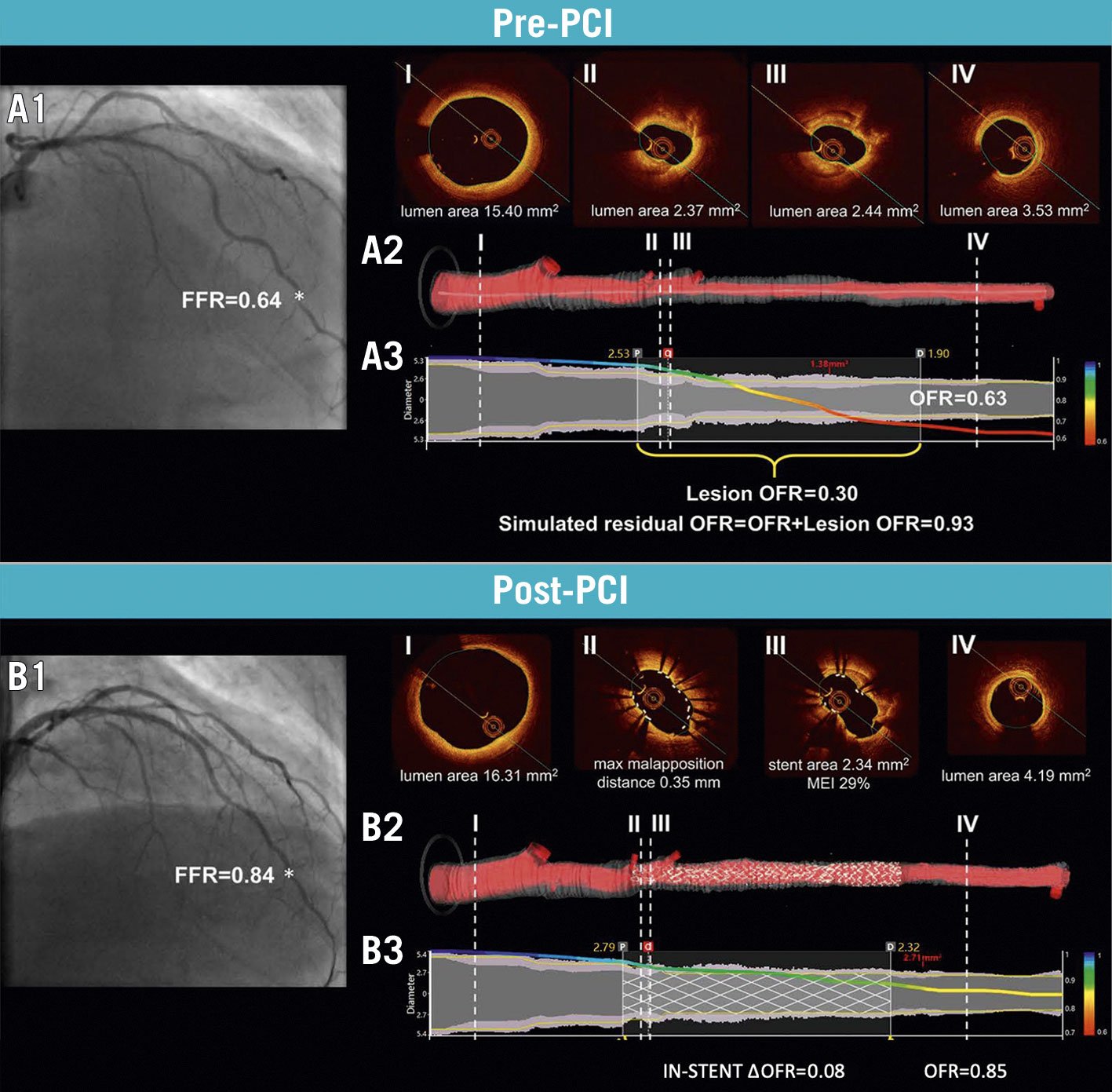
Figure 13. Computation of simulated residual OFR and post-PCI OFR. A1) ICA and FFR value (*) of LAD before PCI. A2) Cross-sectional proximal reference (I), minimum lumen area (II-III), distal reference (IV) and 3D reconstructed artery. A3) The computed OFR along the vessel is presented by a virtual pressure pullback. Vessel pre-PCI OFR is 0.63, with a drop across the lesion of 0.30 and a simulated residual OFR of 0.93. B1) ICA and FFR (*) after PCI. B2) Cross-sectional OCT showing incomplete stent apposition and underexpansion. B3) Post-PCI OFR of 0.85 and virtual OFR pullback showing a diffuse in-stent pressure drop of 0.08. FFR: fractional flow reserve; ICA: invasive coronary angiography; LAD: left anterior descending artery; MEI: minimum expansion index; OCT: optical coherence tomography, OFR: optical flow ratio; PCI: percutaneous coronary intervention. Adapted with permission from Ding et al90.
See ➔ Virtual PCI
Patterns of epicardial atherosclerosis
An accurate PW-pullback allows defitinion of the point-by-point definition of the physiological map along the diseased coronary artery, either at rest or during prolonged hyperaemia9192. A focal pattern of disease is defined as an abrupt pressure drop localised in a relatively short vessel segment (18-20 mm or less), whereas diffuse disease is defined as a gradual reduction of the pressure gradient from distal to proximal, without a clearly identifiable focal pressure drop93. The hyperaemic pullback pressure gradients index (PPGindex) is derived from FFR-pullback and aims to provide a quantitative estimation of the physiological phenotype, rather than relying on a qualitative and operator-dependent interpretation. It is calculated by the magnitude of pressure drop over 20 mm and the overall extent of functional disease, providing a value between 0 to 1. The higher the PPGindex, the more focal the physiological pattern; the lower the PPGindex, the more diffuse the pattern91. A similar index, with analogous computation and values is the QFR virtual pullback index derived from ICA-based computational QFR94. Another approach, dFFR(t)/dt, provides an instantaneous FFR gradient per unit of time. The peak value of dFFR(t)/dt predicts the amount of FFR change across the target stenosis, and, consequently, the expected FFR gain after PCI. The need for motorised FFR-pullback during acquisition and co-registration with ICA represents a limitation to its widespread adoption95.
The physiological pattern of disease should influence the decision-making for appropriate CAD management. In cases of focal stenosis, PCI is the most effective strategy to improve myocardial ischaemia and clinical outcomes. Conversely, in cases of diffuse CAD, either OMT or CABG should be considered, in view of the poor results of “full-metal jacket” stenting approaches9196. A summarising illustration is provided in Figure 5.
See ➔ Discordance, Serial stenoses
Post-PCI physiology
Suboptimal physiology (FFR <0.90) after apparently successful angio-guided PCI is very common (up to 30% of cases) and poorly recognised. Post-PCI physiological evaluations remain underused in clinical practice, mainly due to technical and economic reasons, prolonged procedural times and the need for the administration of hyperaemic agents when wire-based FFR is performed97. LAD localisation, low baseline FFR and the presence of diffuse disease have been associated with an increased risk for suboptimal post-PCI physiological outcomes98. Combined physiology and intracoronary imaging studies have identified several stent-related factors that cause residual intra-stent pressure gradients: geographical miss (failure to stent the full plaque length), stent malapposition, stent underexpansion, major edge dissection, as well as in-stent plaque or thrombus prolapse99. Retrospective and prospective studies have suggested a significant interaction between suboptimal post-PCI physiology and clinical outcomes, with different post-PCI target thresholds (i.e., FFR ≥0.90, iFR ≥0.95 and QFR ≥0.90)100101102103. A comprehensive illustration is provided in Figure 14.
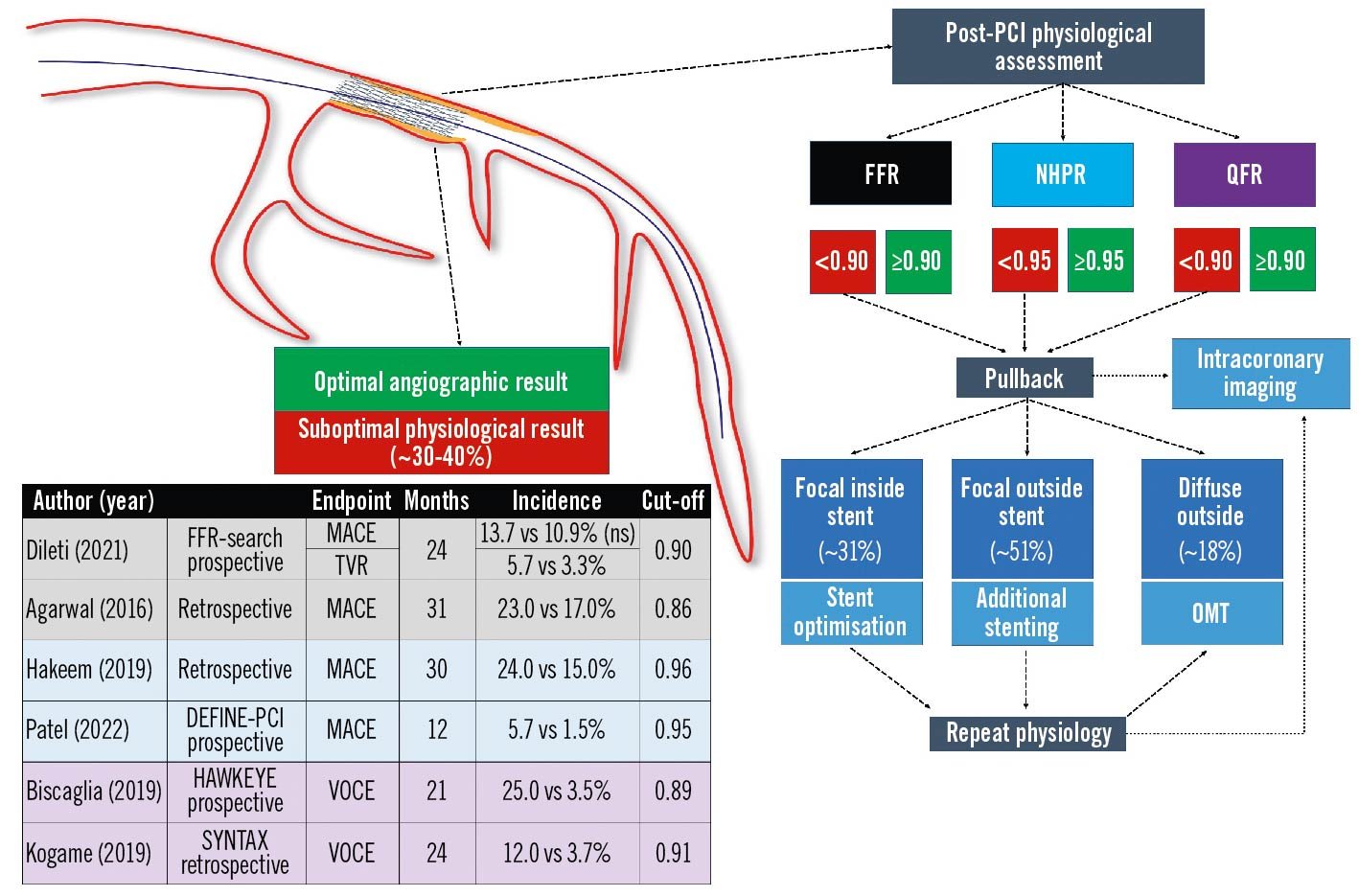
Figure 14. Post-PCI physiology: state-of-the-art. Post-PCI physiological assessment detects suboptimal functional improvement in a large proportion of cases with apparently optimal angiographic results and could have a prognostic relevance in terms of vessel-oriented adverse events. FFR: fractional flow reserve; MACE: major adverse cardiac events; NHPR: non-hyperaemic pressure ratios; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; TVR: target vessel revascularisation; VOCE: vessel-oriented composite endpoint
See ➔ Virtual PCI
QFR
The most validated angio-derived physiological index is quantitative flow ratio (QFR), which computes FFR from the ICA without the need for dedicated PW or hyperaemic stimuli, sharing the same abnormality cut-off (0.80). QFR integrates the 3-dimensional angiographic reconstruction of the vessel, derived from 2 angiographic projections with at least 25° of difference, and coronary flow is estimated from frame counting. QFR can be analysed both online and offline, with a shorter analysis time than FFR (5.0 min vs 7.0 min). Moreover, QFR virtual mapping is inherently co-registered with ICA and has been validated against PW-pullback44. QFR accurately assesses the haemodynamic significance of coronary stenoses compared with FFR and yields nearly optimal accuracy metrics (84% sensitivity, 88% specificity)104105106107. The QFR-guided PCI strategy proved superior to the angio-guided one in the FAVOR III China, sham-controlled RCT108.
Vessel FFR is another index with benefits and disadvantages similar to QFR. Vessel FFR allows a per-lesion analysis rather than requiring a full-length coronary evaluation. No frame counting is needed and hyperaemic flow is derived from the aortic root pressure tracing109. FFRangio allows a full left and right simultaneous coronary artery 3-dimensional vessel reconstruction, based on 3 or more angiographic views. The epicardial and microcirculatory physiology is evaluated as an analogue system whose resistance is derived from the arterial length and diameter. In the FAST-FFR trial, FFRangio yielded good diagnostic accuracy compared to FFR110.
See ➔ Angiography, Normal values, Trials (FAVOR)
Serial stenoses
Physiological evaluation of serial stenoses still represents a challenging problem given the well-known crosstalk phenomenon (Figure 15). Since the pressure drop across a stenosis depends on its severity and flow, the presence of serial stenoses affects overall vessel haemodynamics, especially during maximal hyperaemia. The proximal lesion gradient (interlesion gradient) is underestimated due to the erroneous increase of the Pd value; conversely, the reduced flow due to the proximal stenosis may falsely overestimate the significance of the distal lesion4792111. Hyperaemic PW-pullback represents a pragmatic solution through the identification of the segment with the largest pressure drop, which should be stented first. This approach, however – advocating hyperaemia induction – may overestimate the lesion severity, leading to the treatment of non-significant stenoses92111. In theory, NHPR and NHPR-pullback are less influenced by such phenomena because autoregulation aims at maintaining stable, normal resting coronary flow, which may avoid stenosis crosstalk. In cases of concomitant LM and either LAD or LCx disease, a potential strategy could be the placement of the distal PW in a large unobstructed side branch in order to identify separately the pressure drop caused by the LM disease (the most proximal stenosis) 111.
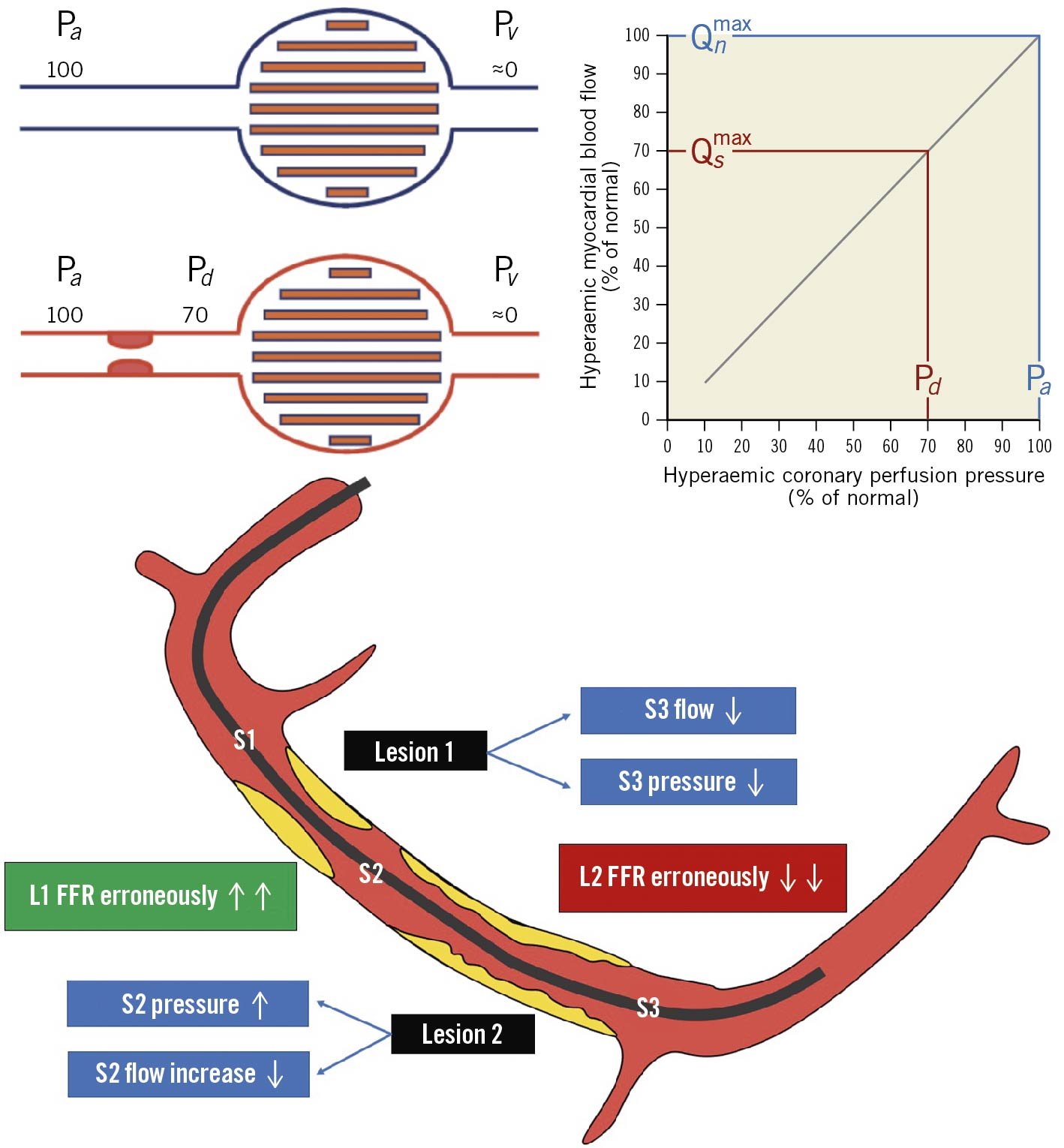
Figure 15. Serial stenoses: the cross-talk phenomenon. Coronary flow physiology is affected by the complex interplay between serial stenoses. The proximal lesion (1) reduces flow and pressure leading to an increase gradient across the distal lesion and FFR reduction (severity overestimation). Conversely the distal lesion might increase the inter-lesion pressure (S2) reducing the pressure gradient across the proximal one, thus leading to FFR increase (severity underestimation). FFR: fractional flow reserve; Pa: aortic pressure; Pd: distal pressure; Pv: venous pressure; Qn: hypothetical maximal myocardial flow without stenosis; Qs: maximal myocardial flow in the presence of stenosis. Adapted with permission from Pijls et al47.
TAVI
The association of severe aortic valve stenosis with CAD, besides being common (25-50%)27, poses diagnostic and therapeutic challenges. The haemodynamic changes due to aortic stenosis raise concerns of the reliability of the hyperaemic and resting physiological indices. Left ventricular hypertrophy is associated with interstitial fibrosis, multivessel disease and increased ventricular diastolic pressures. Conversely, after transcatheter aortic valve implantation (TAVI), an abrupt reduction in afterload and progressive regression of LV hypertrophy occurs, with progressively decreasing resting flow and increasing aortic pressure.
To summarise, observational studies indicate the following 112113114115:
- Pre-TAVI, NHPR tends to be abnormal, while FFR tends to be falsely normal.
- Post-TAVI, NHPR shows erratic individual variations, FFR tends to decrease (Pa increases). However, a change in the initial pre-TAVI decision to defer is rare (decision to treat post-TAVI in <10% of the lesions).
- At follow-up (6-12 months), NHPR may increase significantly with a high rate of lesion reclassification (~30%). FFR remains stable over time if normal at baseline (FFR >0.85) but tends to worsen if borderline at baseline (0.75-0.85).
A comprehensive illustration is provided in Figure 16.
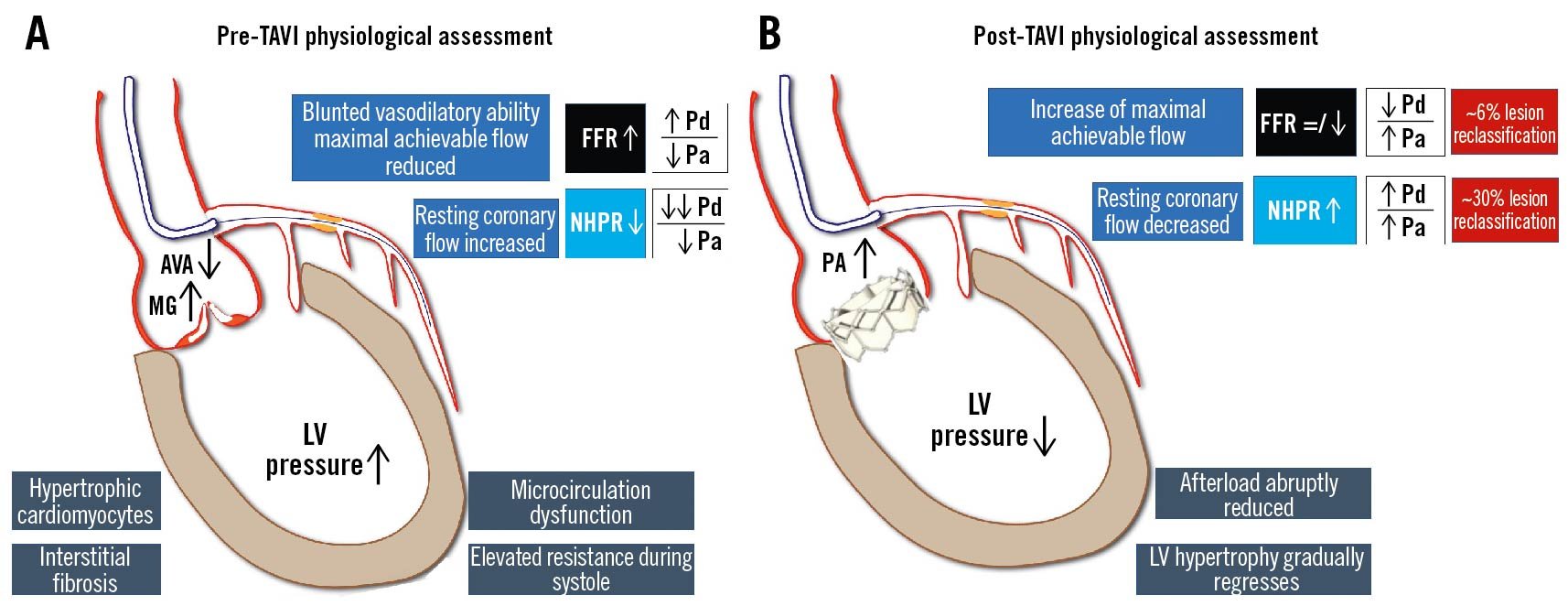
Figure 16. Invasive physiology assessment and aortic stenosis. Interstitial fibrosis and microvascular dysfunction due to aortic stenosis affect both hyperaemic and resting functional indices. A) The increase of LVEDP leads to a rise in resting coronary flow, falsely overestimating NHPR values, while the blunted vasodilatory ability reduces peak hyperaemic coronary flow, underestimating FFR. B) After valve replacement, the reduction of afterload leads to an increase of NHPR values, with negligible impact on FFR values. AVA: aortic valve area; FFR: fractional flow reserve; LV: left ventricular; LVEDP: left ventricular end-diastolic pressure; MG: mean gradient; NHPR: non-hyperaemic pressure ratios; Pa: aortic pressure; Pd: distal pressure; TAVI: transcatheter aortic valve implantation
See ➔ Microcirculation dysfunction
Thermodilution
Thermodilution is an indicator-dilution method that allows the measurement of cardiac output, while coronary thermodilution aims to study microvascular function. CFR is the capacity of coronary flow to increase and is expressed as the ratio between maximum hyperaemic and resting flow. IMR is an indirect estimate of the microvascular resistance obtained during bolus injection thermodilution116.
Continuous thermodilution measures absolute coronary flow (ml/min) and allows a calculation of the total resistance of the myocardial distribution volume downstream from the investigated coronary artery and its components, namely epicardial and microcirculatory resistance. Continuous thermodilution correlates with positron emission tomography-derived flow (ml/min/gr) and resistance, either with or without adenosine infusion 117. A dedicated catheter with 4 side holes (RayFlow; Hexacath) is required in order to induce maximal hyperaemia during continuous saline infusion 116.
See ➔ Microvascular dysfunction
Tips and tricks
Since invasive physiology represents the cornerstone and reference standard for the physiological evaluation of coronary stenosis significance, any technical- or operator-related artefacts should be avoided by proper procedural instrumentation. After IC nitrate administration to avoid vasospasm, accurate equalisation, NHPR/FFR measurements, PW-pullback and final signal drift check must be performed as standard procedural steps. On top of this, iFR coregistration superimposes the physiological map on the angiogram, precisely underlying the area and extent of iFR loss, and identifying the target for PCI (stent location, diameter and length). A comprehensive illustration is provided in Figure 17.
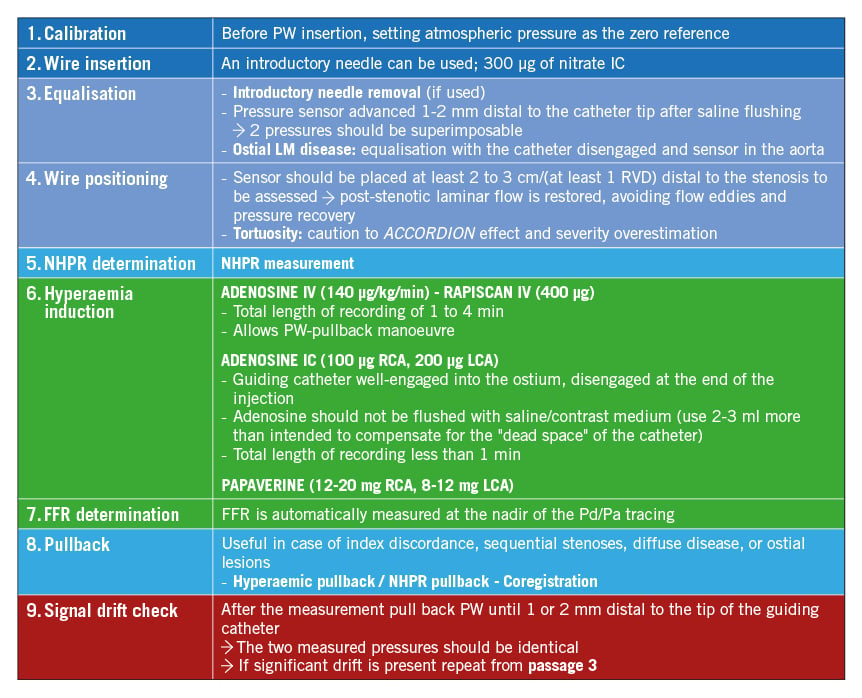
Figure 17. Invasive physiology assessment in the catheterisation laboratory: a practical guide. FFR: fractional flow reserve; IC: intracoronary; IV: intravenous; LCA: left coronary artery; LM: left main; NHPR: non-hyperaemic pressure ratios; Pa: aortic pressure; Pd: distal pressure; PW: pressure wire; RCA: right coronary artery; RVD: reference vessel diameter
See ➔ Hyperaemia
Transplant vasculopathy
Cardiac allograft vasculopathy due to an inflammatory fibroproliferative disease that diffusely affects epicardial coronary arteries and the microcirculation still represents the main drawback of heart transplantation 118. Such pathological alterations affect the reliability of pressure-derived indices in allograft vasculopathy. Indeed, discordant normal FFR and reduced CFR have been reported because the increased microvascular resistance decreases the maximum achievable hyperaemic flow down the epicardial vessel. For this reason, FFR may not provide a reliable index for the revascularisation decision-making in cardiac transplant patients 118119.
Trials
FAME
The FAME I trial provided evidence on the long-term safety of FFR guidance for PCI in patients with multivessel CAD. Patients (n=1,005) were randomly assigned either to FFR-guided PCI (cut-off 0.80) or angio-guided PCI. The use of FFR significantly reduced the number of stents used per patient (p<0.001) and the incidence of a composite endpoint of death, non-fatal MI, repeat revascularisation at 12 months (13.2% vs 18.3%; p=0.02), with loss of statistical significance at 24 months 120 and 5 years of follow-up 121. Treatment deferral with a normal FFR value was safe, with MI and repeated revascularisation rates as low as 0.2% and 3.2% at 2 years, respectively.
The FAME II trial tested the hypothesis that FFR-guided PCI in stable CAD with significant ischaemia would offer better results than OMT alone, reducing the need for urgent, unplanned revascularisation. Patients with an abnormal FFR value (≤0.80) were randomly assigned either to PCI (+OMT) or to OMT alone. The trial was halted prematurely after the enrolment of 1,220 patients, due to a significantly higher incidence of adverse events in the OMT group (12.7% vs 4.3%; p<0.001), exclusively driven by a higher rate of urgent, unplanned revascularisation. The initial sample size and the premature discontinuation of the study did not allow it to achieve relevant conclusions in terms of hard clinical endpoints82.
The FAME III trial was conceived to prove non-inferiority of FFR-guided PCI compared to CABG in case of three-vessel CAD, using last-generation drug-eluting stents. The trial failed to meet its primary composite endpoint at 12 months (all-cause death, MI, stroke and revascularisation) with 10.6% for FFR-guided PCI versus 6.9% for CABG (p for non-inferiority=0.35). FFR-guided PCI was associated with a lower incidence of major bleeding, acute kidney injury, arrhythmia, and rehospitalisation within 30 days and with a shorter length of hospital stay. Additional insights on the safety and efficacy of FFR-guided PCI were provided when applied in the subgroup of low-risk patients (SYNTAX score <23) 122.
DEFINE-FLAIR and iFR-SWEDEHEART
The DEFINE-FLAIR and the iFR SWEDEHEART were two large RCTs that tested the non-inferiority of iFR compared to FFR in guiding revascularisation. Both these trials included stable CAD and non-culprit lesions of ACS, enrolling 2,492 and 2,037 patients, respectively. In both trials, iFR provided non-inferior results compared to FFR in terms of MACE at 12-month follow-up (6.8% vs 7.0%; p for non-inferiority<0.001 in DEFINE-FLAIR and 6.7% vs 6.1%; p for non-inferiority=0.007 in iFR SWEDEHEART). More patients reported intraprocedural chest discomfort in cases of FFR use, compared to iFR 123124. Moreover, iFR resulted in increased deferral rates compared to FFR (45% vs 50%; p<0.01), according to a pooled meta-analysis of both trials. Revascularisation deferral was safe in both groups with no difference in 12-month MACE (4.12% vs 4.05%, respectively; p=0.60) 125. Of note, long-term follow-up data, of particular importance in case of deferral, have been reported only for the iFR SWEDEHEART, showing no difference in the 5-year MACE incidence between the iFR and FFR groups: 21.5% and 19.9%, respectively (hazard ratio [HR] 1.09, 95% confidence interval [CI]: 0.90-1.33) 126.
FAVOR
The FAVOR (Functional Diagnostic Accuracy of Quantitative Flow Ratio in Online Assessment of Coronary Stenosis) Pilot, FAVOR II Europe-Japan and FAVOR II China studies showed that QFR improves the diagnostic accuracy of ICA in predicting abnormal FFR values104105106.
The FAVOR III China randomised sham-controlled trial showed that a QFR-guided PCI strategy is superior to an angio-guided one, with a significant reduction of MACE at 12-month follow-up (5.8% vs 8.8%; HR 0.65, 95% CI: 0.51-0.83; p=0.0004). Moreover, QFR permitted a change in procedural plans in more than 1 out of 5 cases. As observed with FFR-guidance, QFR-guidance resulted in a smaller number of stents implanted, a shorter procedural time and a lower volume of contrast use108.
See ➔ Guidelines
UFR
Ultrasonic flow ratio (UFR) is an IVUS-derived FFR that combines the morphological anatomical features of greyscale IVUS with functional mapping through computational fluid dynamics. UFR has shown strong correlation and optimal agreement with invasive FFR analysis (sensitivity 92%; specificity 91%; [+] predictive value 96%; [-] predictive value 96%)65127. The main drawback of the initial IVUS-derived FFR models was the long simulation time (>1 hour). Recently, UFR has shown a fast computational time (~100 sec) with excellent reproducibility.
See ➔ Virtual PCI
Virtual PCI
The concept of virtual PCI relies on the pre-PCI reconstruction of the physiological map of the stenosed vessel in order to achieve optimal post-PCI physiology through individualised procedural planning. PW-pullback recordings allow the operator to precisely define the functional phenotype of the stenotic vessel and to anticipate the physiological effect of the fully deployed stent of selected length and diameter, with the help of augmented reality. For instance, iFR gain (iFRexp) showed a good correlation with the observed iFR post-PCI 128, even in cases of diffuse and multiple lesions. In the iFR GRADIENT registry, the iFR-assisted strategy led to a change in the revascularisation strategy in 1 out of 3 cases, reducing the number of lesions and the diseased length treated93. Similarly, QFR computation provided a virtual haemodynamic mapping of the coronary artery that has shown excellent agreement with iFR-pullback as a reference44. Moreover, pre-PCI QFR demonstrated excellent accuracy in predicting post-PCI results, with both estimated and measured residual-QFR being well correlated with actual post-PCI FFR, and adverse clinical events at follow-up129130.
To precisely allocate the physiological blockages on the anatomical angiographic map along the vessel, a system of angiography-physiology coregistration has been developed (Figure 18). Such innovation has provided promising results for PCI planning and guidance, especially with diffuse disease or multiple stenoses in series131. A case example of virtual PCI is provided in Figure 19.
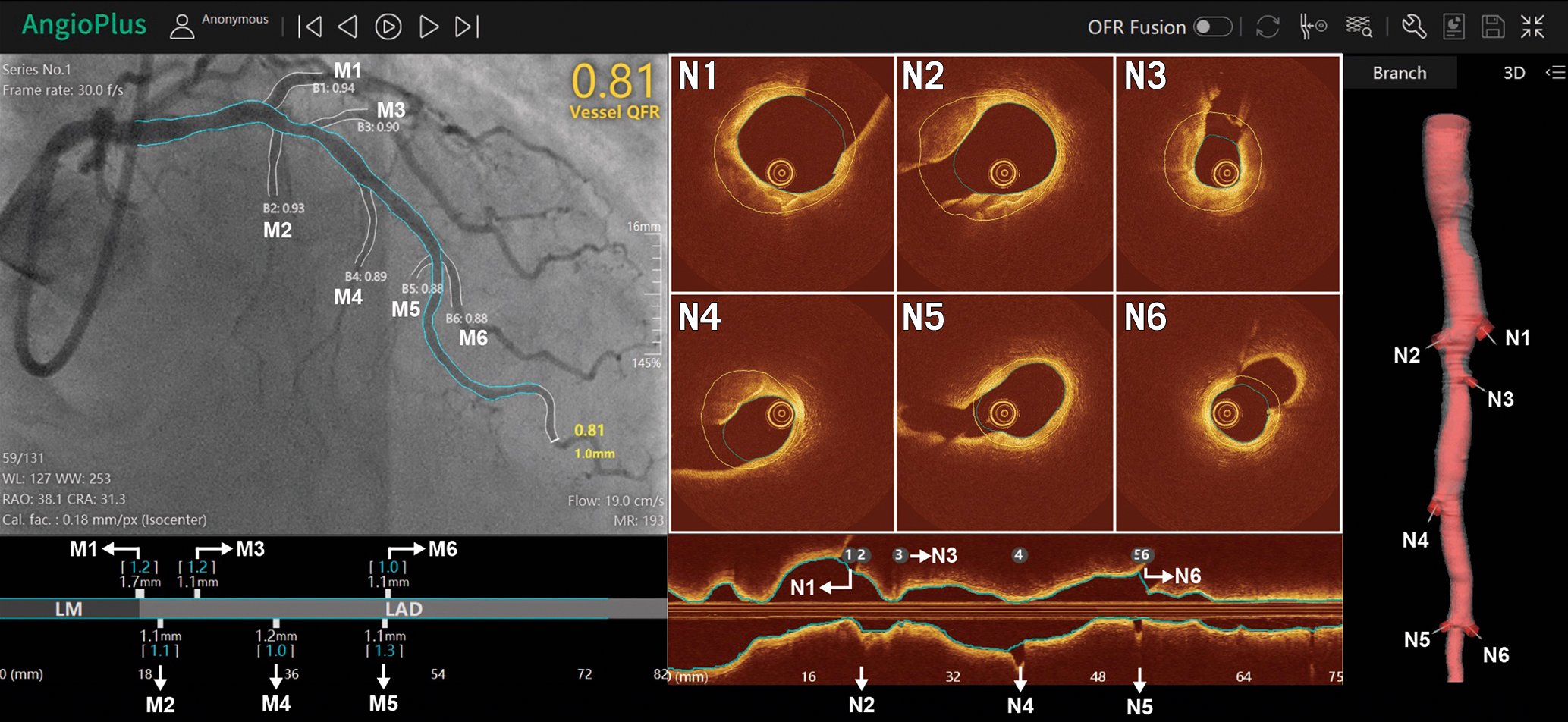
Figure 18. Physiology-imaging angiography coregistration: a step forward towards precision medicine. M1 to M6 and N1 to N6 are the side branches detected on angiography and OCT images, respectively. All side branches are automatically and accurately matched by the software (AngioPlus, version 3.0, Pulse Medical Imaging Technology), where M1 to M6 correspond to N1 to N6, respectively. OCT: optical coherence tomography; QFR: quantitative flow ratio
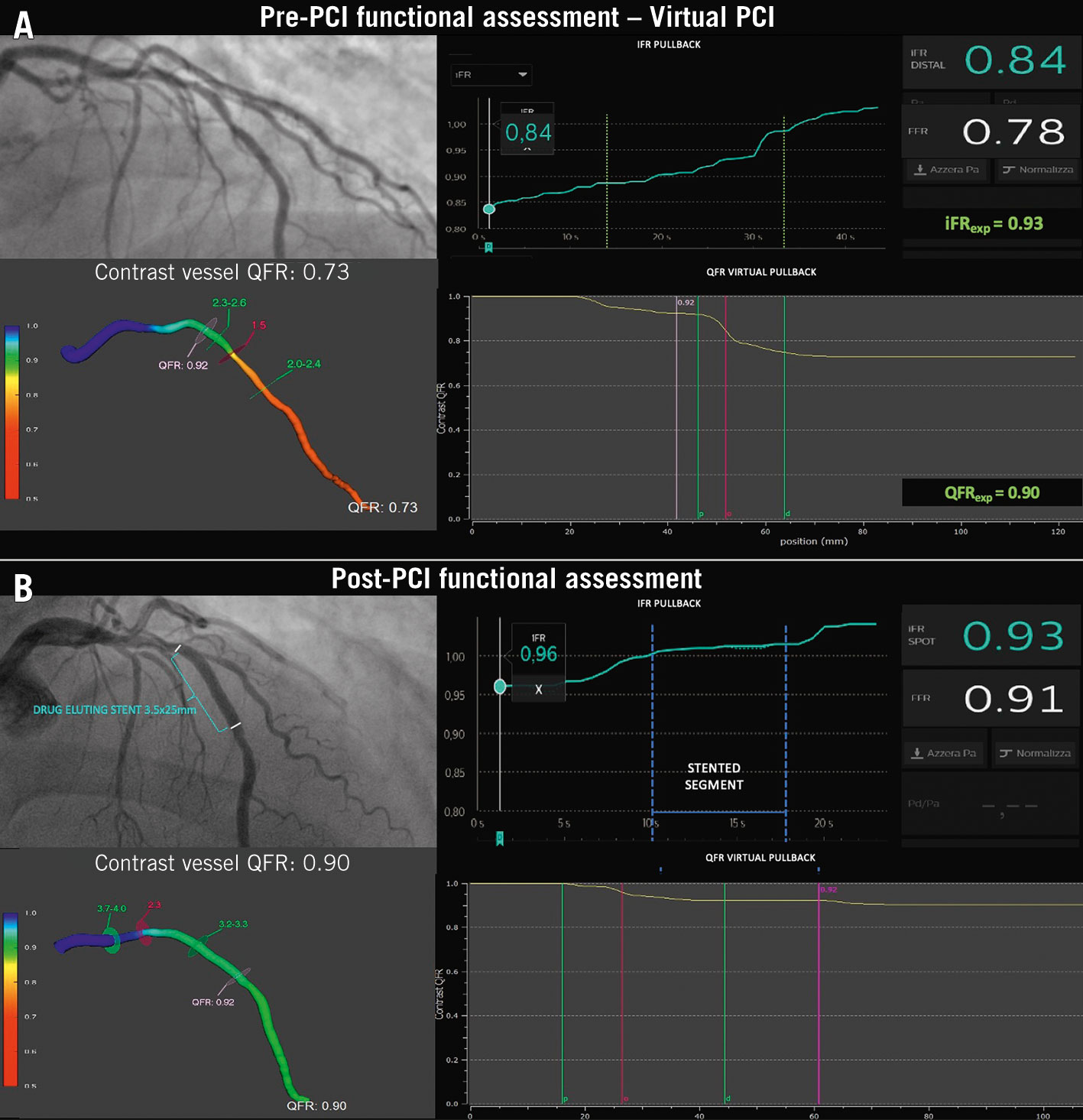
Figure 19. Virtual PCI: a case example. A) The combined FFR, iFR and QFR assessment of a mid-LAD stenosis identifying a significant reduction of coronary flow (FFR 0.78; iFR 0.84; QFR 0.73) and a mixed pattern at the iFR/QFR-pullback (a focal drop in the mid segment with diffuse disease proximally and distally). The expected iFR and QFR after the focal lesion treatment were 0.93 and 0.90, respectively. B) Repeated physiology assessment post-PCI providing acceptable results (FFR 0.91, iFR 0.96 and QFR 0.90). The residual diffuse disease proximally and distally to the stented segment are confirmed at the PW- and QFR-pullback. FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; LAD: left anterior descending; PCI: percutaneous coronary intervention; PW: pressure wire; QFR: quantitative flow ratio
See ➔ Post-PCI physiology
Wires and catheters
Any size of 6 Fr or larger guiding catheter can be used for physiological assessment. The aortic waveform should display the dicrotic notch precisely. Size mismatch between the catheter and coronary ostium may impact on coronary flow, preventing maximal hyperaemic flow response with an erroneously increased FFR and underestimation of disease severity. Any ventricularisation of the pressure waveform has to be recognised and avoided. Saline flushing before FFR measurement allows elimination of any residual contrast material inside the catheter. Both guiding catheters with side holes and diagnostic catheters should be avoided for FFR measurements, since they do not provide a reliable aortic pressure waveform46, hyperaemic agents may not be delivered intracoronarily, and smaller guides do not allow intervention should a complication occur.
Many pressure-measuring systems are available, of which a number are listed below:
- 0.014” wires with a sensor (electric or fibre optic) at 3-3.5 cm from the distal radiopaque tip can currently be used as regular guide-wires for PCI intervention: PressureWire (St. Jude Medical); WaveWire (Philips); OptoWire (Opsens Medical); Comet Pressure Guidewire (Boston Scientific).
- 0.020” microcatheters with a pressure sensor (fibre optic) mounted on a common guidewire: Navvus (Acist Medical Systems). Even thin microcatheters may impede maximal hyperaemic coronary flow, potentially underestimating FFR values, especially in the presence of mild disease. At the same time, these devices may offer advantages in cases of complex PCI such as bifurcation lesions (SB assessment)132.
See ➔ Tips and tricks
Acknowledgements
The authors are very thankful to Mona Tirèn who provides healthcare professionals with an outstanding literature resource about coronary physiology in her monthly “Coronary Physiology News” messages.
Funding
This work was supported in part by the grants from Science Foundation Ireland (15/RP/2765) to D. Ding, S. Fezzi, J. Huang, M. Lunardi and W. Wijns and by grants from the Natural Science Foundation of China (No. 82020108015 and 81871460) to S. Tu.
Conflict of interest statement
W. Wijns received a research grant and honoraria from MicroPort (TARGET AC trial); is the co-founder of Argonauts, an innovation facilitator; and is the senior medical advisor at Rede Optimus Research and Corrib Core Laboratory. S. Tu is a consultant for and received research grants from Pulse Medical. The other authors have no conflicts of interest to declare.

