Antonios Halapas1*, MD, PhD, FESC; Michael Chrissocheris1, MD, PhD; Gregory Pattakos2, MD, MS; Panagiota Kourkoveli1, MD, PhD; Kostas Papadopoulos1, MD, FESC; Panos Vardas1, MD, PhD, FESC; Kostas Spargias1, MD, PhD, FESC
AsiaIntervention 2019;5:149-152, DOI: 10.4244/AIJ-D-18-00058
1. Department of Transcatheter Heart Valves, Hygeia Hospital, Athens, Greece; 2. Department of Cardiothoracic Surgery, Hygeia Hospital, Athens, Greece
Abstract
Treatment of a failing aortic bioprosthesis by transcatheter valve-in-valve (ViV) therapy has become an alternative to redo surgery. However, the ViV technique may be less effective in small surgical valves because of patient/prosthesis mismatch (PPM). Here we will discuss the bioprosthetic valve fracture/remodelling (BVF) procedure and the most important issues regarding this promising new technique.
Abbreviations
AVA: aortic valve area
AV-Vmax: aortic valve peak velocity
BPV: bioprosthetic valve
BVF: bioprosthetic valve fracture
CABG: coronary artery bypass grafting
CAF: chronic atrial fibrillation
COPD: chronic obstructive pulmonary disease
GA: general anaesthesia
HOR: hybrid operating room
ICU: intensive care unit
ID: inner diameter
MDSCT: multidetector spiral computed tomography
MG: mean gradient
MR: mitral regurgitation
LAD: left anterior descending artery
LVEF: left ventricular ejection fraction
OD: outer diameter
OM: obtuse marginal
PG: peak gradient
PPM: patient/prosthesis mismatch
sAVR: surgical aortic valve replacement
SOV: sinus of Valsalva
sPAP: systolic pulmonary artery pressure
TAVR: transcatheter aortic valve replacement
TEE: transoesophageal echocardiography
TF: transfemoral
ViV: valve-in-valve
Introduction
Treatment of a failing bioprosthesis by transcatheter valve-invalve (ViV) therapy has become an alternative to redo surgery. However, ViV treatment is problematic with small (≤21 mm in diameter) surgical bioprostheses because of a further reduction in the effective valve orifice1. One way to overcome this challenge may be to fracture the ring of the surgical valve by high-pressure balloon dilatation after implanting a transcatheter aortic valve2.
Methods
An 84-year-old Caucasian male was referred to our Heart Team due to severe symptomatic bioprosthetic aortic valve deterioration. Symptoms included shortness of breath and fatigue, and the patient was in New York Heart Association (NYHA) functional Class III heart failure. His past medical history included: coronary artery bypass grafting (CABG) (x3, 2012) combined with surgical aortic valve replacement (sAVR) with a biological stented Mosaic® A19 valve (Medtronic, Minneapolis, MN, USA). Baseline echocardiography post sAVR revealed a peak gradient (PG) of 36 mmHg. Comorbidities included: chronic atrial fibrillation (CAF) with a CHA2DS2-VASc score of 4 on anticoagulation therapy, insulin-dependent diabetes mellitus, arterial hypertension, dyslipidaemia and mild chronic obstructive pulmonary disease (COPD). The last echocardiography (16/05/2018) showed a severely degenerated aortic bioprosthesis with an estimated aortic valve area (AVA) of 0.6 cm2, aortic valve peak velocity (AV-Vmax) of 4.3 m/s, mean gradient (MG) of 50 mmHg and PG of 74 mmHg with preserved left ventricular ejection fraction (LVEF) (65%). Moreover, he had moderate eccentric mitral regurgitation (MR) and severe pulmonary hypertension with a systolic pulmonary artery pressure (sPAP) of 55 mmHg. The recent coronary angiography (14/05/2018) showed two-vessel disease with patent grafts to the left anterior descending (LAD) and obtuse marginal (OM) branches (OM1 and OM2). In electrocardiogram (ECG)-gated cardiac multidetector spiral computed tomography (MDSCT) with contrast agent (19/4/2018), the inner diameter (ID) of the aortic bioprosthesis was 14.5 mm and the outer diameter (OD) was 18 mm. Moreover, the diameter of the sinus of Valsalva (SOV) was 30x30x26 mm and the coronary height was more than 1 cm. The femoroiliac system was suitable for transfemoral (TF) approach. The calculated logistic EuroSCORE was 25.10%, the Society of Thoracic Surgeons (STS) mortality score was 9% and the STS morbidity/mortality score was 35%. Based on the abovementioned medical history and the high surgical risk, our Heart Team decided to proceed with TF valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) using the bioprosthetic valve fracture (BVF) technique. The procedure was performed in our hybrid operating room (HOR) with the patient placed in supine position, under general anaesthetic (GA) and transoesophageal echocardiography (TEE) guidance. Antibiotics were administered intravenously before the procedure and intravenous heparin 50 U/ kg was administered during the procedure targeting an activated clotting time of 250 seconds. Access to the left common femoral artery and vein was obtained. Access to the contralateral right femoral artery was obtained and the preclose technique was performed with two ProGlide® devices (Abbott Vascular, Santa Clara, CA, USA). A 14 Fr eSheath (Edwards Lifesciences, Irvine, CA, USA) was introduced. A temporary pacing wire was placed in the apex of the right ventricle. According to the size and brand of the bioprosthesis, the ViV true ID was predicted to be 16 mm. However, on 3mensio (Pie Medical Imaging BV, Maastricht, the Netherlands) analysis, the ID was 14.5 mm due to tissue growth around the valve inflow area. Our strategy was to proceed initially with ViV TAVR using a SAPIEN 3 20 mm valve (Edwards Lifesciences) and possibly proceed with BVF if there were to be a residual elevated MG across the valve. This was indeed the case, as there remained a 13 mmHg MG across the aortic annulus. The decision was taken to perform BVF. An Atlas Gold Balloon Dilatation Catheter (CR Bard Inc., Tempe, AZ, USA) 18 mm system was selected. The size of the high-pressure post balloon diameter was decided according to the true ID of the bioprosthesis. A high-pressure stopcock was used to attach a syringe and an Indeflator (Edwards Lifesciences) separately to the balloon. With the stopcock open to the syringe, an initial hand inflation was performed to inflate the balloon rapidly. Then the stopcock was opened to the Indeflator, and the pressure was gradually increased (up to 10 atm) in the balloon system until the BPV ring fractured.
Results
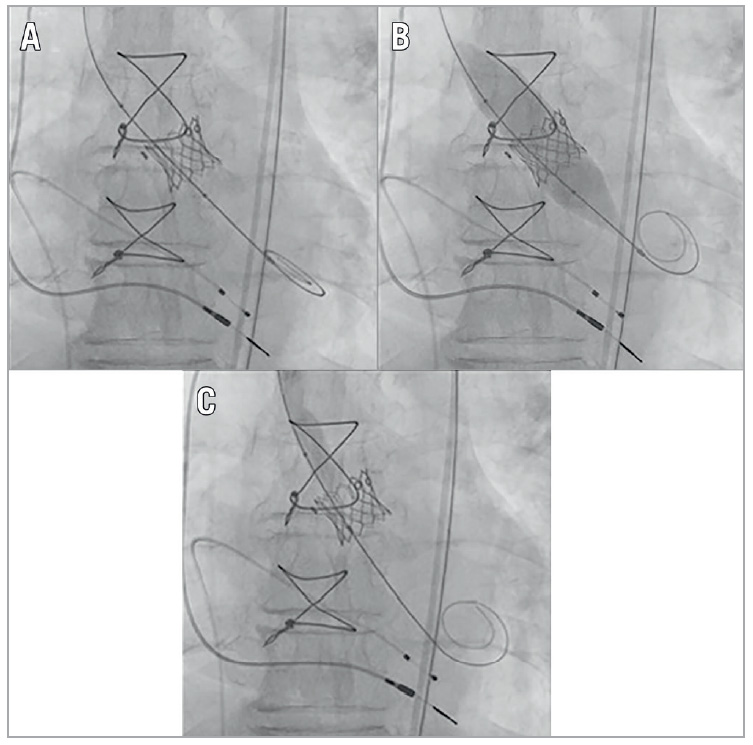
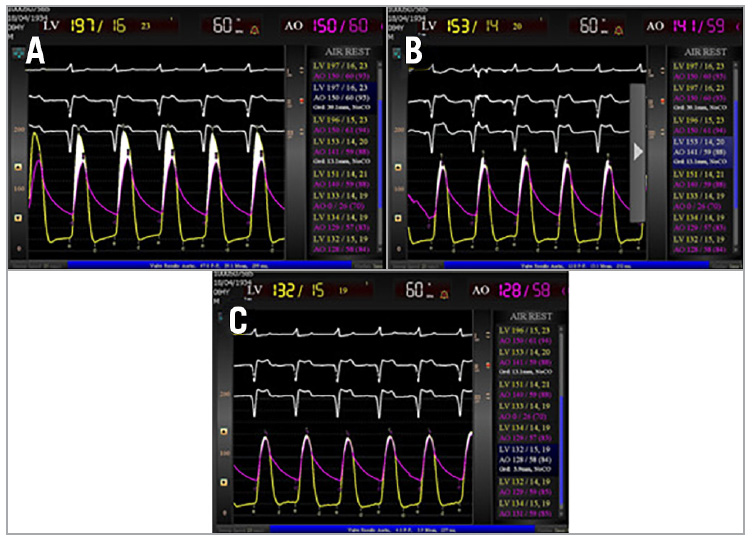
Fracture of the BPV ring was noted with a sudden decrease in inflation pressure and the visible release of the balloon waist (Figure 1). The MG was improved from 39 mmHg at baseline to 13 mmHg post ViV and 3 mmHg post BVF (Figure 2). After sheath removal and ProGlide closure, the peripheral entry integrity was checked by angiography. The fluoroscopy time was 25 minutes and the volume of contrast agent was 80 mL. The patient was extubated immediately post procedure in the HOR and transferred to the intensive care unit (ICU). On postoperative day one, the patient was transferred to normal station and subsequently discharged on day four.
Figure 1. Fluoroscopic images of the stages of valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR) followed by bioprosthetic valve fracture (BVF). A) Immediately after ViV TAVR. B) During BVF before fracture of the surgical ring. Note the waist of the balloon at the level of the surgical valve ring. C) Final fluoroscopic results.
Discussion
In the aortic position severe PPM occurs in between 2% and 20% of the cases. A recent meta-analysis suggested that predictors of PPM post sAVR include: older age, female sex, hypertension, diabetes mellitus, renal failure, larger body surface area, larger body mass index, and the utilisation of a bioprosthesis1. Furthermore, the presence of PPM is prognostically important because PPM results in higher valve gradients, increasing perioperative and overall mortality1. Several strategies have been developed to avoid PPM following ViV TAVR. Recent publications have reported on the concept of fracturing the surgical BPV ring with a high-pressure balloon inflation in order to dilate the BPV and permit further expansion of the THV, thereby improving haemodynamic results2.
At present, ViV TAVR BVF indications are not fully defined. The majority of patients, in particular those with large surgical valves, are likely to achieve an adequate haemodynamic result with ViV TAVR, and patients without PPM following ViV TAVR have an excellent survival to one year3. Therefore, patients who stand to benefit the most from BVF are those who are predisposed to PPM and high residual MGs following ViV TAVR, including those with a small (≤21 mm) bioprosthesis and/or stenosis as the mechanism of BPV failure3. Whether patients with large BPVs (>21 mm labelled valve size) or intermediate MGs (10-20 mmHg) after ViV TAVR stand to benefit from BVF is not known. In our case the MG was further improved from 13 mmHg (intermediate gradient) post ViV to 3 mmHg post BVF (Figure 2).
An important question remains as to the timing of BVF, that is, before or after TAVR. There are potential advantages of both strategies. Fracture of the bioprosthetic ring pre TAVR may allow the use of a larger-sized TAVR prosthesis. On the other hand, although BVF post TAVR may allow further expansion of the TAVR valve, there is a risk that the leaflet may tear, resulting in aortic insufficiency, as well as potential dislodgement of the fractured valve and embolisation of debris. Also, when BVF follows TAVR, the TAVR prosthesis itself is subjected to a high-pressure balloon dilatation, which in some cases may cause acute structural damage or accelerated degeneration. In either scenario, BVF may reduce PPM and optimise haemodynamics by decreasing the residual aortic valve gradient during ViV TAVR. Whether the timing of BVF is a determinant of clinical outcomes remains to be seen4.
Finally, due to concern that BVF may result in aortic root injury or coronary artery obstruction, some operators have preferred to perform BVF only in the setting of full haemodynamic backup with extracorporeal membrane oxygenation (ECMO). To date, no published reports of aortic root injury or haemodynamic collapse attributable to BVF exist5.
Figure 2. Example of procedural haemodynamics with ViV transcatheter aortic valve replacement and bioprosthetic valve fracture. A) Baseline (pre-ViV) haemodynamics demonstrating severe bioprosthetic aortic stenosis with a mean aortic valve gradient of 36 mmHg. B) Haemodynamics post ViV with SAPIEN 3 20 mm implantation revealed a mean gradient of 13 mmHg. C) Haemodynamics post BVF demonstrating a mean gradient of 3 mmHg.
Limitations
Whether BVF has an impact on the survival of patients who are at risk of PPM following ViV TAVR remains to be seen. Further data are needed as to the quality of life benefit that is gained from ViV TAVR with BVF compared with ViV TAVR alone. The feasibility of BVF in patients with larger BPVs has not been well studied. Finally, the safety margins for performing BVF in patients at risk of coronary obstruction and aortic root injury are not fully understood and warrant further study.
Conclusions
BVF can be an effective and safe procedure in small surgical valves to facilitate ViV TAVR by balloon-expandable or selfexpanding transcatheter valves, resulting in reduced residual transvalvular gradients and increased valve effective orifice area.
Impact on daily practice
ViV treatment is problematic with a small surgical bioprosthesis because of a further reduction in the effective valve orifice. Bioprosthetic valve fracture using a high-pressure balloon dilatation can be safely performed to facilitate ViV.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Dayan V, Vignolo G, Soca G, Paganini JJ, Brusich D, Pibarot P. Predictors and Outcomes of Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc Imaging. 2016; 9:924-33.
2. Nielsen-Kudsk JE, Christiansen EH, Terkelsen CJ, Nørgaard BL, Jensen KT, Krusell LR, Tang M, Terp K, Klaaborg KE, Andersen HR. Fracturing the Ring of Small Mitroflow Bioprostheses by High-Pressure Balloon Predilatation in Transcatheter Aortic Valve-In-Valve Implantation. Circ Cardiovasc Interv. 2015;8: e002667.
3. Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC, Leipsic J, Kodali SK, Makkar R, Miller DC, Pibarot P, Pichard A, Satler LF, Svensson L, Alu MC, Suri RM, Leon MB. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-In- Valve Registry. J Am Coll Cardiol. 2017;69:2253-62.
4. Allen KB, Chhatriwalla AK, Cohen DJ, Saxon JT, Aggarwal S, Hart A, Baron S, Davis JR, Pak AF, Dvir D, Borkon AM. Bioprosthetic Valve Fracture to Facilitate Transcatheter Valve-In- Valve Implantation. Ann Thorac Surg. 2017;104:1501-8.
5. Saxon JT, Allen KB, Cohen DJ, Chhatriwalla AK. Bioprosthetic Valve Fracture During Valve-in-valve TAVR: Bench to Bedside. Interv Cardiol. 2018;13:20-6.
To download, please click below.