Vinayak Nagaraja1, MBBS, MS, MMed (Clin Epi), FRACP; Stuart Moss2, MBBS; Nigel S. Jepson1, BMedSci, MBBS, FRACP; Mark Pitney3*, MBBS, FRACP
AsiaIntervention 2019;5:27-31, DOI: 10.4244/AIJ-D-18-00020
1. Department of Cardiology, Prince of Wales Hospital, Sydney, Australia; 2. St George Hospital, Sydney, Australia; 3. Sutherland Heart Clinic, Sutherland Hospital, Sydney, Australia
Introduction
Bioresorbable vascular scaffolds (BVS)1-3 were designed to be an effective substitute to conventional metallic stents and overcome limitations including positive vessel remodelling, stent fracture, neoatherosclerosis, being a perpetual nidus for stent thrombosis and inhibition of physiological vasodilation4,5. The most widely studied polymeric devices comprise poly-L-lactide (PLLA) struts coated with poly-D, L-lactide (PDLLA) and an antiproliferative agent4,5. Although late outcomes with the Absorb™ bioresorbable vascular scaffold (Abbott Vascular, Santa Clara, CA, USA) are disappointing6, this only confirms the need to gain a better understanding of the late behaviour of these scaffolds. The literature so far suggests that there is some evidence supporting their use in venous grafts7-10. This case description illustrates the unique findings of optical coherence tomography (OCT) imaging of a BVS in a vein graft at 40 months post implantation.
Methods
A 77-year-old lady had previously undergone two bypass operations, the latter following recurrent restenoses of conventional metallic stents. Approximately seven years post second bypass (April 2013), she had symptoms (angina) with inducible ischaemia on a stress echocardiogram. Angiography found high-grade proximal disease in a saphenous venous graft to the left anterior descending artery. The lesion was adequately prepared using a 3.0 mm predilatation balloon (Tazuna® [Terumo Corp., Tokyo, Japan] – a semi-compliant percutaneous transluminal coronary angioplasty [PTCA] balloon catheter). A 3.5×28 mm Absorb BVS was deployed at 14 atmospheres and post-dilation was performed with a 3.75 mm non-compliant balloon (Hiryu® [Terumo Corp.] – a non-compliant PTCA balloon catheter) to 21 atmospheres. A distal protection device was utilised (3.0 mm SpiderFX™ [Medtronic, Minneapolis, MN, USA] embolic protection device) and the patient was placed on dual antiplatelet therapy for six months (aspirin 100 mg daily and clopidogrel 75 mg daily).
Results
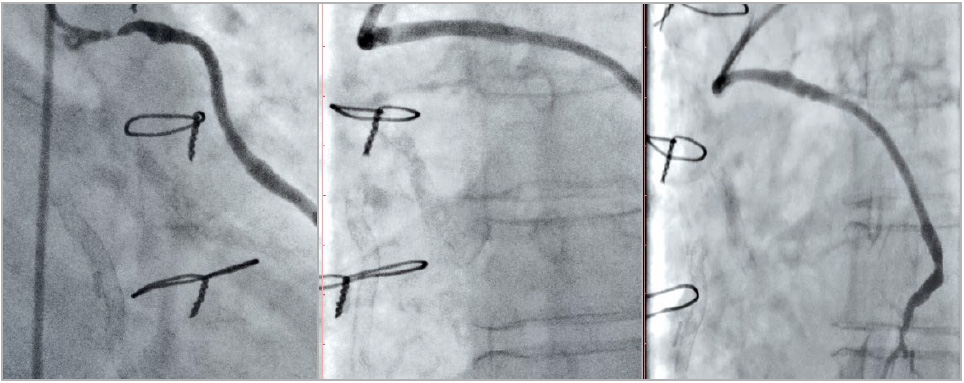
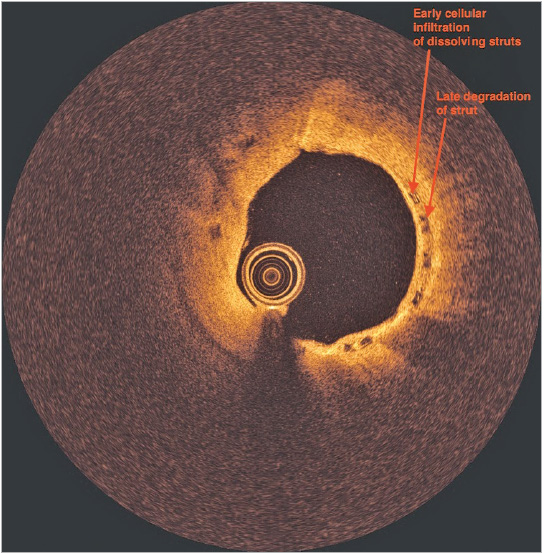
Approximately 40 months post implant, she again developed symptoms (angina); an exercise test was abnormal and repeat coronary angiography was performed. This demonstrated new flow-limiting disease in a small to medium-sized native marginal circumflex artery, successfully treated with plain balloon angioplasty using a 2.0 mm predilatation balloon (Tazuna). Given concerns as to whether the diseased circumflex artery explained her presentation and the fact that the proximal segment of the vein graft to the left anterior descending artery appeared to have reduced volume (Figure 1), an OCT study (Moving image 1, Figure 2) was performed to assess the BVS in the vein graft to the left anterior descending artery. This demonstrated good apposition of the scaffold, tissue coverage with a neointimal depth of 0.48 mm and five uncovered struts. There were several struts manifesting various stages of PLLA degradation ranging from early cellular infiltration of dissolving struts (very high peak intensity at the core with low median intensity) to late degradation of the strut (moderately high peak intensity with a high median intensity). No intervention was performed on the vein graft. Post procedure, she was placed on dual antiplatelet therapy for six months (aspirin 100 mg daily and clopidogrel 75 mg daily) and has remained well since.
Discussion
In September 2017, Abbott Vascular ceased supplying BVS, citing low demand but more likely due to disappointing clinical results. They also signalled their intention to release a newer improved device for future clinical trials. Accepting that initial poor implantation technique and inappropriate vessel selection played a role, the four-year ABSORB II data6 showed a statistically higher rate of target lesion failure (TLF) (11.1% vs. 5.6%). There were no cases of stent/scaffold thrombosis between three and four years and some see this as the potential start of scaffold benefit. Data do support a “learning curve”: the three-year data of ABSORB III11 showed only a trend towards a higher rate of TLF in the ABSORB arm (13.4% vs. 10.4%) but with a persistently higher rate of device thrombosis (2.3% vs. 0.7%), whilst the 30-day ABSORB IV (using an optimal implantation technique) reported reduced early events (30-day scaffold thrombosis reduced from 1.1% in ABSORB III to 0.4% in ABSORB IV at 30 days) to the extent that ABSORB IV shows non-inferiority at 30 days. Given that a “learning curve” probably exists, cases such as this and any knowledge gained remain clinically relevant.
The development of a “vascular restoration” strategy in the form of BVS has been considered by many as the “fourth revolution” in the percutaneous management of coronary artery disease12,13. So far, seven trials have been published comparing everolimus-eluting bioresorbable scaffolds and everolimus-eluting metallic stents14,15. The benefit of bioresorbable stents is thought to pay off in the long term: at around twelve months there is a loss of mechanical support that allows conditioning of tissue to enhance healing16, and at three years1 full reabsorption is noted, that assists in physiological function and neo-media formation in coronary arteries. Based on trial evidence at 60 months17, the BVS struts disappear completely.
Recently, Nakatani and colleagues18 performed an intravascular ultrasound echogenicity analysis and light intensity analysis on OCT in a porcine model with a follow-up duration of 48 months. There was good correlation observed for the strut depolymerisation process and integration process post comprehensive bioresorption. In addition, late luminal enlargement was observed at three to four years and was associated with strut integration.
Some bioresorbable stents are still commercially available; however, we are still awaiting the results of ABSORB IV, due for release in the next few years. Newer-generation stents such as the Magmaris™ (Biotronik, Bülach, Switzerland) are showing promising results after publication of BIOSOLVE II and III19, in which improved stent and study design adds strength to the argument that BVS may still play a role in future percutaneous interventions.
Figure 1. Saphenous vein graft to the left anterior descending artery pre implantation, immediately post implantation of the BVS and at 40 months post implantation of the BVS. BVS: bioresorbable vascular scaffold
Figure 2. Optical coherence tomography image of the BVS.
Limitations
The current study has a few limitations. Data were collected from an observational study and might be subject to potential bias. ACS was defined according to current guidelines. However, the method of case identification may not have been completely specific, because patients with RI are more likely to have elevated levels of cardiac markers. The GFR estimated at admission reflected the renal function before any intervention during the index hospitalisation. The renal function was not re-evaluated pre-discharge or during follow-up and thus worsening or improvement of renal function could not be ruled out. Larger studies are needed to define better the impact on renal dysfunction, especially in those with severe RI and/or on haemodialysis, in all ACS patients, whether they are treated conservatively or by an invasive strategy, and also to re-evaluate renal function during the study course.
Conclusions
In this Middle Eastern patient population who had PCI, the presence of moderate to severe RI was an independent predictor for in-hospital and one-year cardiac mortality and major bleeding events. Estimating the GFR in such a population is strongly recommended in order to define better high-risk groups among these patients such that vigilance can be exercised for adverse cardiovascular events.
Impact on daily practice
The relatively low incidence rate of adverse cardiovascular events in a PCI-exclusive group should encourage healthcare professionals to refer ACS patients with RI to an invasive strategy.
Funding
The study was supported by an unrestricted grant from AstraZeneca.
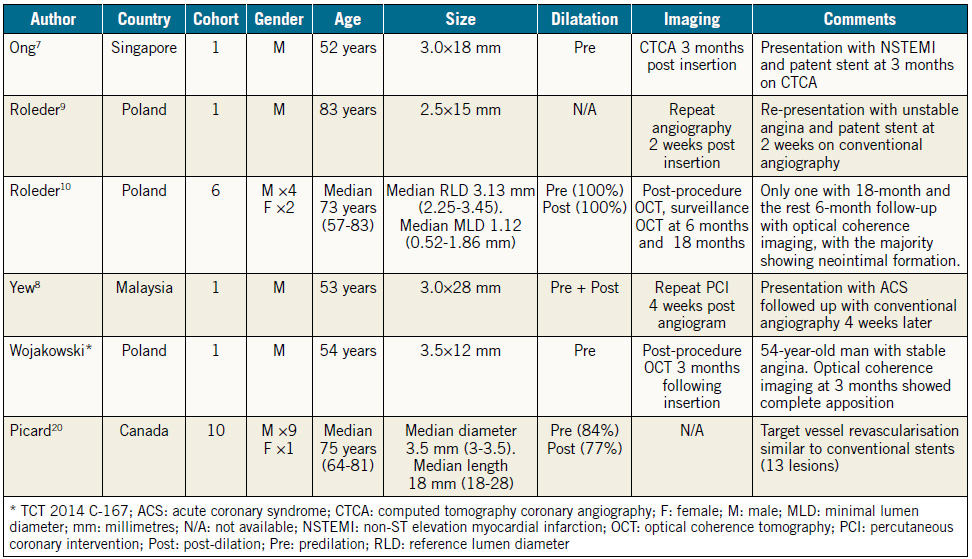
Table 1. Summary of bioresorbable vascular scaffolds in saphenous venous grafts.
Moving image 1. Optical coherence tomography study of the saphenous vein graft bioresorbable vascular scaffold.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM, Regar E, Kamberi M, Powers JC, Rapoza R, van Beusekom H, van der Giessen W, Virmani R. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. 2010;122:2288-300.
2. Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Miquel-Hebert K, Rapoza R, García-García HM. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122: 2301-12.
3. Abizaid A, Ribamar Costa J Jr, Bartorelli AL, Whitbourn R, van Geuns RJ, Chevalier B, Patel T, Seth A, Stuteville M, Dorange C, Cheong WF, Sudhir K, Serruys PW; ABSORB EXTEND investigators. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10:1396-401.
4. Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R, Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899-907.
5. Ormiston JA, Serruys PW. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009;2:255-60.
6. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrié D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida M, Campo G, Iñiguez A, Sabaté M, Windecker S, Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-91.
7. Ong PJ, Jafary FH, Ho HH. “First-in-man” use of bioresorbable vascular scaffold in saphenous vein graft. EuroIntervention. 2013;9:165.
8. Yew KL. Novel use of absorb bioresorbable vascular scaffold and STENTYS self-apposing coronary stent for complex saphenous vein grafts intervention. Int J Cardiol. 2014;177:e184-5.
9. Roleder T, Ochala A, Smolka G, Wanha W, Wojakowski W. Implantation of a bioabsorbable vascular scaffold into a coronary vein graft: a two-week angiography follow-up. Kardiol Pol. 2014; 72:281.
10. Roleder T, Wanha W, Smolka G, Zimoch J, Ochala A, Wojakowski W. Bioresorbable vascular scaffolds in saphenous vein grafts (data from OCTOPUS registry). Postepy Kardiol Interwencyjnej. 2015;11:323-6.
11. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW; ABSORB III Investigators. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J Am Coll Cardiol. 2017;70:2852-62.
12. Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation. 2011;123:779-97.
13. Wykrzykowska JJ, Onuma Y, Serruys PW. Vascular restoration therapy: the fourth revolution in interventional cardiology and the ultimate “rosy” prophecy. EuroIntervention. 2009;5 Suppl F: F7-8.
14. Sorrentino S, Giustino G, Mehran R, Kini AS, Sharma SK, Faggioni M, Farhan S, Vogel B, Indolfi C, Dangas GD. Everolimus- Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J Am Coll Cardiol. 2017;69:3055-66.
15. Zhang XL, Zhu QQ, Kang LN, Li XL, Xu B. Mid- and Long-Term Outcome Comparisons of Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;167:642-54.
16. Gogas BD, Benham JJ, Hsu S, Sheehy A, Lefer DJ, Goodchild TT, Polhemus DJ, Bouchi YH, Hung OY, Yoo SY, Joshi U, Giddens DP, Veneziani A, Quyyumi A, Rapoza R, King SB 3rd, Samady H. Vasomotor Function Comparative Assessment at 1 and 2 Years Following Implantation of the Absorb Everolimus-Eluting Bioresorbable Vascular Scaffold and the Xience V Everolimus-Eluting Metallic Stent in Porcine Coronary Arteries: Insights From In Vivo Angiography, Ex Vivo Assessment, and Gene Analysis at the Stented/Scaffolded Segments and the Proximal and Distal Edges. JACC Cardiovasc Interv. 2016;9: 728-41.
17. Serruys PW, Ormiston J, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, Chevalier B, Smits P, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Wasungu L, Ediebah D, Veldhof S, Onuma Y. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis: 5-Year Follow-Up. J Am Coll Cardiol. 2016;67: 766-76.
18. Nakatani S, Ishibashi Y, Sotomi Y, Perkins L, Eggermont J, Grundeken MJ, Dijkstra J, Rapoza R, Virmani R, Serruys PW, Onuma Y. Bioresorption and Vessel Wall Integration of a Fully Bioresorbable Polymeric Everolimus-Eluting Scaffold: Optical Coherence Tomography, Intravascular Ultrasound, and Histological Study in a Porcine Model With 4-Year Follow-Up. JACC Cardiovasc Interv. 2016;9:838-51.
19. Haude M, Ince H, Kische S, Abizaid A, Tölg R, Alves Lemos P, Van Mieghem NM, Verheye S, von Birgelen C, Christiansen EH, Barbato E, Garcia-Garcia HM, Waksman R; BIOSOLVE- II and III investigators. Safety and clinical performance of a drug eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries: Pooled 12-month outcomes of BIOSOLVE-II and BIOSOLVE-III. Catheter Cardiovasc Interv. 2018;92:E502-11.
20. Picard F, Marquis-Gravel G, Avram R, Ly HQ, Dorval JF, Doucet S, de Hemptinne Q, L’allier PL, Tanguay JF. Everolimuseluting bioresorbable vascular scaffold implantation to treat saphenous vein graft disease, single-center initial experience. J Interv Cardiol. 2017;30:433-9.
21. Tanaka A, Latib A, Kawamoto H, Jabbour RJ, Sato K, Miyazaki T, Naganuma T, Mangieri A, Pagnesi M, Montalto C, Chieffo A, Carlino M, Montorfano M, Colombo A. Clinical outcomes of a real-world cohort following bioresorbable vascular scaffold implantation utilising an optimised implantation strategy. EuroIntervention. 2017;12:1730-7.
22. Oberhauser JP, Hossainy S, Rapoza RJ. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention. 2009;5 Suppl F:F15-22.
To download, please click below.