Kean H. Soon1*, MBBS, PhD; Nynke H.M. Kooistra2, MD; Michiel Voskuil2, MD, PhD; Adriaan O. Kraaijeveld2, MD, PhD; Pieter R. Stella2, MD, PhD
AsiaIntervention 2019;5:142-148, DOI: 10.4244/AIJ-D-18-00054
1. Department of Medicine-Western Health and Epworth Clinical School, The University of Melbourne, Melbourne, Australia;
2. Hart en Longen, Cardiologie, Universitair Medisch Centrum Utrecht, Utrecht, the Netherlands
Abstract
Aims: We aimed to evaluate the extent of left ventricular (LV) recovery post transcatheter aortic valve implantation (TAVI) and its clinical predictors.
Methods and results: This was a retrospective study on patients treated with TAVI from August 2008 to September 2017. Patients were sub-classified according to their baseline LV function as normal, mildly impaired, moderately impaired or severely impaired. Echo pre TAVI and early post TAVI were compared to assess LV function change. Predictors of LV function change were sought from univariate and multivariate ordinal logistic regression analyses. There were 662 patients included in this study. Nearly half of them, 323 patients (49%), had abnormal LV systolic dysfunction of various degrees. Of these, 193 (60%) showed LV function improvement post TAVI. Based on their pre-TAVI LV function, 55% of the mild LV dysfunction cohort, 62% of the moderate LV dysfunction cohort and 74% of the severe LV dysfunction cohort had LV function improvement post TAVI. Multivariate logistic regression analysis revealed baseline LV dysfunction as the only significant predictor of LV function improvement post TAVI.
Conclusions: The majority of patients with baseline LV dysfunction had LV improvement post TAVI, more so those patients with severe LV dysfunction.
Abbreviations
AS: aortic stenosis
EF: ejection fraction
LV: left ventricular
LVF: left ventricular failure
LVFn: left ventricular function
NYHA: New York Heart Association
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) is becoming the treatment of choice for intermediate- and high-risk patients with severe aortic stenosis (AS). TAVI is non-inferior to surgical aortic valve replacement (SAVR) in terms of perioperative mortality and stroke rate; it is associated with a lower incidence of acute kidney injury, atrial fibrillation and shorter recovery time, albeit at the expense of increased pacemaker implantation rate and vascular complications1,2. Among patients with severe AS undergoing TAVI, about one third of them have left ventricular systolic failure (LVF) with ejection fraction (EF) lower than 50%3. The prognosis of patients with reduced left ventricular (LV) function is worse than that of those with preserved LV function, whether they are treated with TAVI or SAVR4-6. Concerns about its prognosis and uncertainty about the possibility of LV recovery may lead to reluctance of TAVI operators to accept severe AS patients with poor LV function for TAVI. Our study aimed to assess the extent and time course of LV function recovery post TAVI. We also aimed to identify factors that might predict the reversibility of LVF dysfunction in the setting of severe AS after TAVI.
Methods
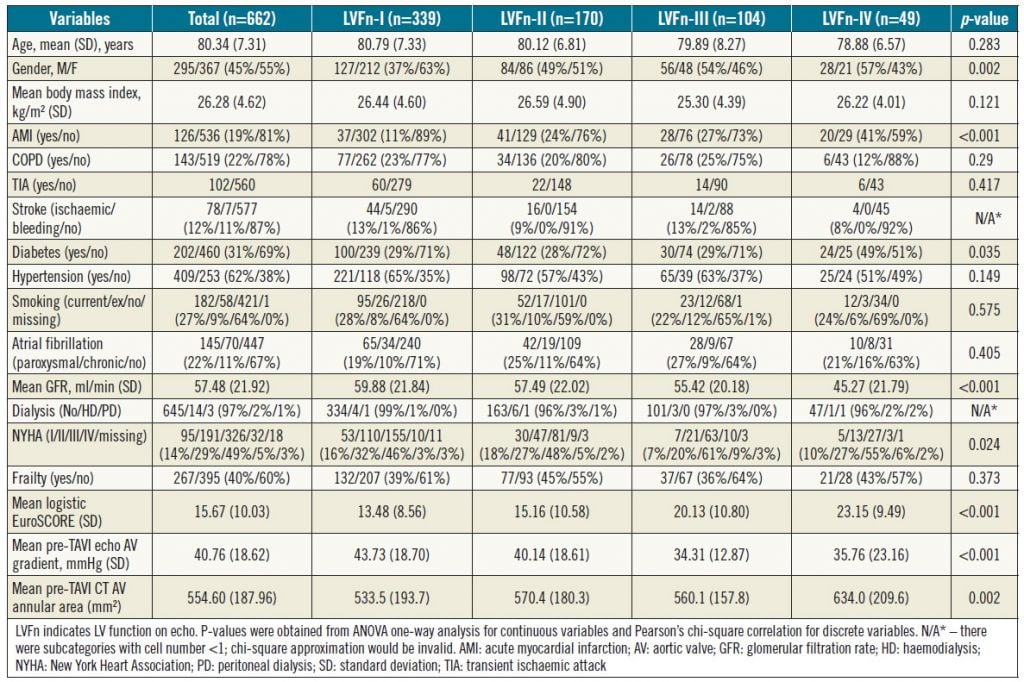
This was a retrospective study carried out at a tertiary cardiac centre with an established structural heart intervention programme. All cases of TAVI performed between August 2008 and September 2017 were included in the study. The inclusion criterion was TAVI in the defined period. Exclusion criteria were absence of echo pre TAVI or within a week post TAVI, and cases that were lost to follow-up. Patient demographics, cardiovascular risk factors, comorbidities, and the findings of pre-TAVI and post-TAVI echo within a week were used for analysis and comparison. LVF was defined as mild if the EF was 45-54% (grade II), moderate if the EF was 30-44% (grade III) and severe if the EF was <30% (grade IV). The outcome of interest was improvement of LV function by at least one grade. Clinical variables and imaging findings were summarised in mean and standard deviation for continuous variables and count and proportion for categorical variables for the whole cohort and subgroups of LV function (Table 1). The differences in these clinical variables among the subgroups of LV function were analysed using one-way analysis of variance (one-way ANOVA) for continuous variables and the chi-square test for discrete variables (Table 1). To identify predictors of LV function change post TAVI, univariate ordinal logistic regression analysis was performed first by setting the order of outcomes as “LV improvement”, “no change” and “LV deterioration”. Variables with a p-value <0.05 were grouped together for subsequent multivariate ordinal logistic regression analysis to identify independent predictors of LV function change.
In the subgroup analysis of patients with severe LVF (grade IV), descriptive analysis was performed in the same manner as described above. Univariate binary logistic regression analysis was performed first to identify predictors of LV improvement in this subgroup. Only those variables with a p-value <0.05 from the univariate analysis were subsequently used in multivariate analysis to identify independent predictors of LV function improvement in this subgroup. All the statistics performed in this study were conducted using the statistics software Minitab® 18 (Minitab LLC, State College, PA, USA).
The patients were followed up by clinic visit at intervals of one month, six months, twelve months and yearly at our centre or at the referring hospital. The study complied with the Declaration of Helsinki and ethics committee approval from our institution was waived given the non-experimental design of the study. All data collected were anonymised before analysis.
Results
In total, 678 consecutive patients were treated with TAVI in the predefined period. Of these, 662 patients with complete data were included in the current analysis (Table 1). Among those not included in the analysis were 11 in-hospital deaths due to complications of bleeding (n=6), tamponade (n=2), stroke (n=2), and pneumonia (n=1); four of these patients had normal grade-I LV function (LVFn) pre TAVI, three patients had grade-II LVFn, three patients had grade-III LVFn and one patient had grade-IV LVFn. Hence, all the in-patient deaths were not statistically skewed towards the cohort with severe LV dysfunction. The characteristics of the 662 patients are summarised in Table 1 according to their baseline LVFn. Overall, those patients with poorer LVFn were more likely to be male, diabetic, renally impaired, symptomatic with higher NYHA class, and with a smaller aortic valve gradient due to reduced LV contractility (Table 1).
LV FUNCTION CHANGE POST TAVI
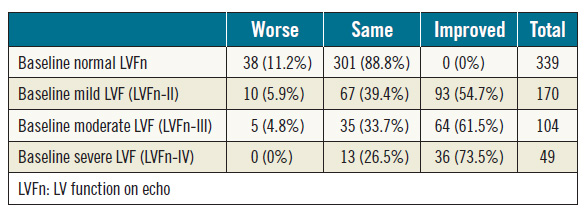
Of the 662 patients included in the analysis, 339 patients (51%) had normal baseline LVFn whereas 323 patients (49%) had abnormal LVFn of various degrees (Table 2). Among 323 patients with abnormal LVFn at baseline, 193 patients (60%) had LVFn improvement post TAVI. Of patients with mild LV dysfunction (grade-II) at baseline, 55% (93/170) had LVFn improvement post TAVI. Of those with moderate LV dysfunction (grade-III), 61% (64/104) had LVFn improvement post TAVI: 42% (44/104) improved by one grade and 19% (20/104) improved by two grades. Of those with severe LV dysfunction (grade-IV), 73% (36/49) improved in their LVFn post TAVI: 55% (27/49) had LV improvement by one grade, 10% (5/49) had LVFn improvement by two grades and 8% (4/49) had improvement by three grades. Interestingly, of those with normal LVFn at baseline, 11% had deteriorated LVFn (Table 2).
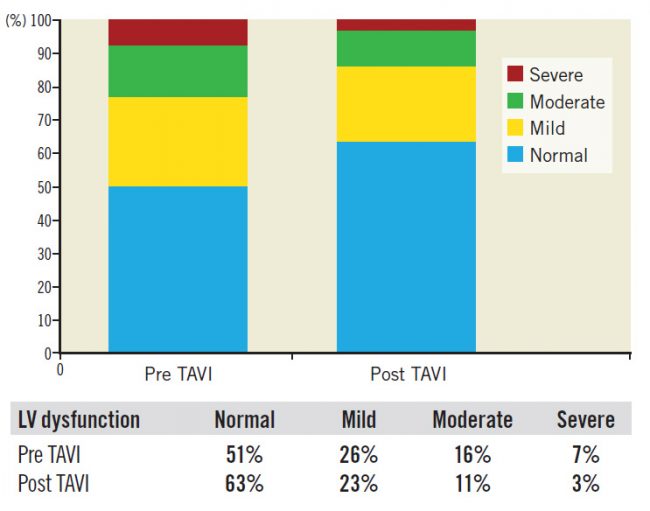
For the whole study cohort (662 patients), 193 patients (29%) had an improved LVFn early post TAVI; 416 patients (63%) had no change in their LVFn while 53 patients (8%) had a deteriorated LVFn (Figure 1).
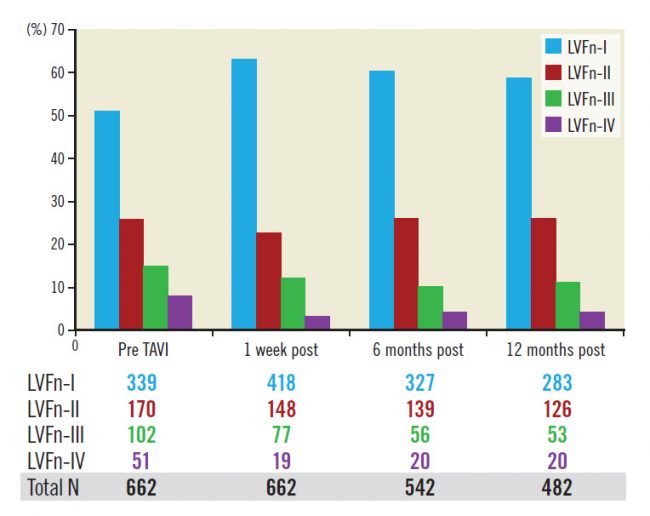
The improvement of LVFn occurred shortly after valve implantation and appeared to be persistent with time as the fraction of LVFn in each LVFn subgroup at baseline remained similar up to 12 months after the procedure (Figure 2).
Table 1. Patients’ clinical, echo and CT variables versus their baseline LV function (LVFn)
Table 2. Baseline LV function versus change of LV function post TAVI; chi-square p<0.001.
Figure 1. Distribution of LV function pre and post TAVI.
PREDICTORS FOR LV FUNCTION CHANGE IN THE WHOLE COHORT
Based on the univariate ordinal logistic regression analysis, only logistic EuroSCORE, pre-TAVI mitral regurgitation (graded), pre-TAVI right ventricular systolic pressure (continuous variable) and LV dysfunction (graded) and post-TAVI pacemaker implantation were identified as significant predictors of LVFn change. Further multivariate analysis of these predictors resulted in identification of LV dysfunction as the only independent clinical predictor of LV change (Table 3).
SEVERE LV DYSFUNCTION SUBGROUP
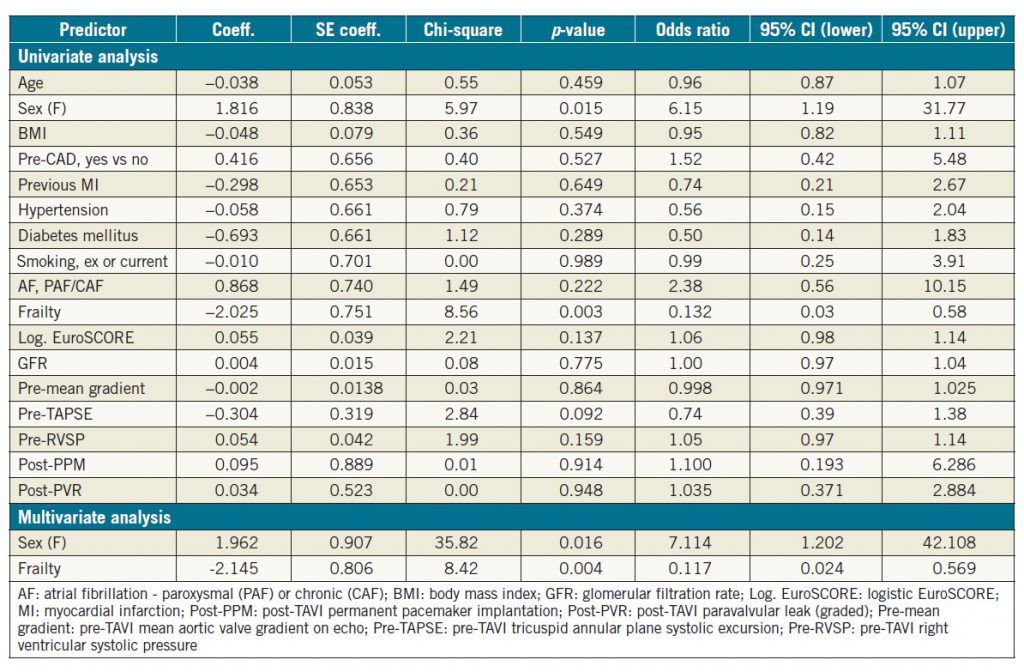
In the subgroup analysis of patients with severe LV dysfunction pre TAVI, only gender and frailty were identified as significant predictors of LV change based on univariate binary logistic regression analysis (Table 4); female gender was a favourable variable for LV improvement whereas increased frailty was less likely to be associated with improved LVFn post TAVI (Table 4). Both variables remained significant predictors despite correcting for each other on further multivariate analysis (Table 4).
FOLLOW-UP
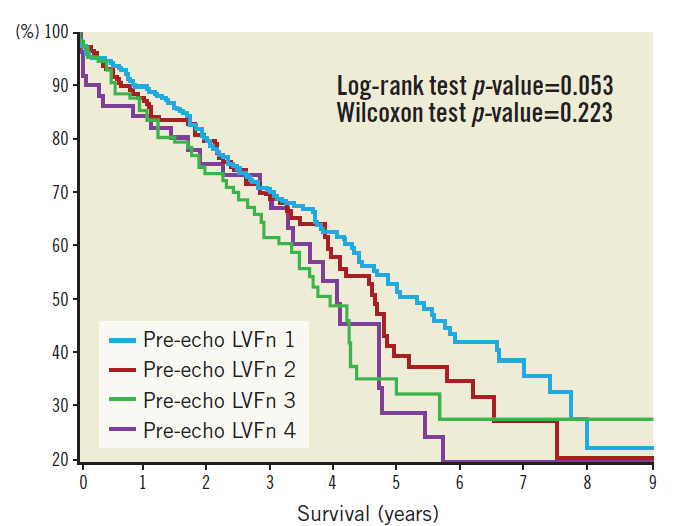
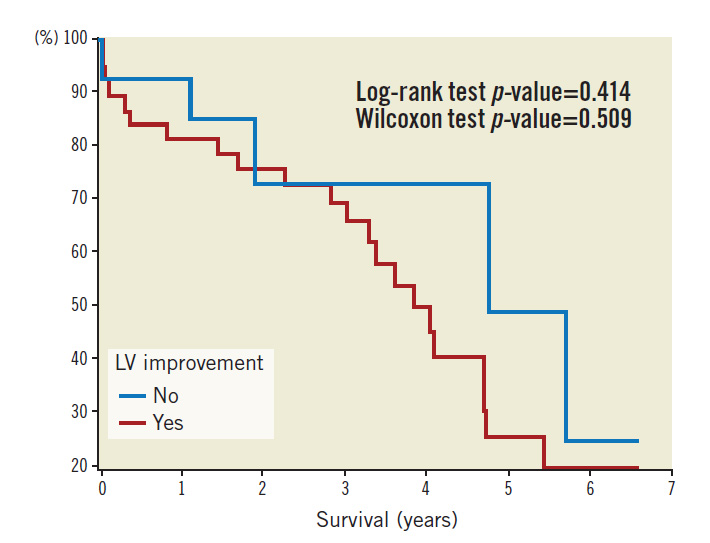
Patients were followed up over a median period of 962 days. Kaplan-Meier survival plots based on pre-TAVI echo LVFn are shown in Figure 3. Kaplan-Meier survival plots for subsets of severe LV dysfunction (with or without LV improvement) are shown in Figure 4. Statistics of the survival plots indicate that the differences among the subgroups of pre-TAVI LVFn were not significant (log-rank p-value=0.053 and Wilcoxon test p-value=0.223). Nonetheless, there was a trend showing that in the long term the separation among the curves became wider; those with a better baseline LVFn might have a better survival probability. Similarly, in the cohort of severe LV failure (LVFn class IV), there was no significant difference in survival probability between those with LV improvement versus those without LV improvement post TAVI (log-rank test p-value=0.414 and Wilcoxon test p-value=0.509).
Table 3. Univariate and multivariate ordinal logistic regression analysis for LV improvement, no change or deterioration post TAVI.
Figure 2. Fractions of LV function class (percentage) pre-TAVI, within a week, six months and twelve months post TAVI remain the same.
Discussion
In our large cohort of patients undergoing TAVI, almost half of them had LV systolic dysfunction of various degrees. Our study showed that more than half of these patients with LV dysfunction had a prompt improvement in their LV systolic function post TAVI. Interestingly, a larger proportion of patients with severe LV systolic dysfunction showed an improvement in LVFn after TAVI in comparison with the subgroups of patients with milder LV systolic dysfunction at baseline. Our findings concur with the findings of a study by Elhmidi et al in which, of patients with severe LV dysfunction, 15% improved to normal EF and 66% improved to mild-moderate dysfunction post TAVI7.
The LV improvement present at one week after TAVI appeared to be sustained during subsequent follow-up. Sustained LVFn improvement is potentially associated with a better survival rate in comparison to those with no LVFn improvement. In a study using LV global longitudinal strain (GLS) as a marker of LVFn improvement, Sato et al demonstrated that such an improvement of LV was sustained even during follow-up over five years. Patients with improved GLS were less likely to die in comparison with those who failed to demonstrate improvement in GLS post TAVI8. Similarly, in the PARTNER-1 trial, the mortality rate of patients with no LVFn recovery after 30 days was twice as high as the mortality rate of those with LV recovery during two-year follow-up3.
Identifying those likely to have LV improvement post TAVI among the patients with baseline LV systolic dysfunction would be an important stratification process, particularly when the resources for TAVI are limited. In our attempt to do so, we could only identify baseline LV systolic dysfunction as the only independent predictor. Elhmidi et al also found that baseline LV systolic dysfunction was the strongest predictor of LV recovery post TAVI7. Contrary to the findings of Freixa et al, our study did not show that coronary artery disease and previous myocardial infarction were significant predictors of failure of LV improvement9.
In the subgroup of patients with severe baseline LV systolic dysfunction, only female gender was identified as a significant predictor of LV function improvement post TAVI, whereas the presence of frailty predicted a low likelihood of LV recovery. Interestingly, in a study comparing TAVR versus SAVR in terms of LV recovery post procedure, Clavel et al also reported that one of the independent predictors of LV recovery was female gender10. The reason for this finding remains to be elucidated.
Table 4. Univariate and multivariate binary logistic regression analysis for LV improvement post TAVI in severe LV dysfunction subgroup.
Figure 3. Kaplan-Meier survival plot as per baseline LV function.
Figure 4. Kaplan-Meier survival plot in the cohort of severe LV failure.
Limitations
A major limitation of our study is the lack of dobutamine stress echo data. Several studies have shown that dobutamine stress echo can predict LV recovery post aortic valve replacement5,6. SAVR patients with no contractile reserve appear to have a much higher operative mortality rate in comparison to patients with contractile reserve, 32% vs. 6%, respectively. In a small study looking at 49 patients with a low gradient type of severe AS, Hayek et al reported a higher mortality in the cohort with no contractile reserve versus those with contractile reserve during in-hospital stay, at 30 days, one year and two years post TAVI11. On the other hand, in the recently published TOPAS-TAVI registry, absence of contractile reserve on dobutamine stress echo did not negate the possibility of LV function improvement post TAVI in those with low-flow low-gradient type of severe aortic stenosis (LFLG-AS); it also failed to predict the mortality outcome of the LFLG-AS patients post TAVI. Hence, the value of pre-TAVI dobutamine stress echo is still questionable12.
As this was a retrospective observational study it has other limitations including missing data, observer and patient selection bias. Not all patients with poor LV systolic function were included in this study due to significant comorbidities and frailty. A randomised controlled study on patients with severe LV dysfunction would help to address these issues.
Conclusions
In our large cohort of patients with severe AS and LV systolic dysfunction, nearly two thirds of the patients had improvement in LV function post TAVI. LV function improvement occurred in a greater proportion of patients with more severe LV dysfunction. Patients should not be excluded from TAVI treatment based on the extent of pre-TAVI LV dysfunction alone.
Impact on daily practice
Patients with severe aortic stenosis and severe LV systolic dysfunction potentially benefit the most from TAVI. They should not be discriminated from receiving TAVI treatment based on their baseline LV dysfunction.
Acknowledgements
Cardiovascular research staff, Hart en Longen, Cardiologie, Universitair Medisch Centrum Utrecht, Utrecht, the Netherlands, and A/Prof. Dean McKenzie, Epworth Healthcare, Richmond, Victoria, Australia.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-31.
2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-20.
3. Elmariah S, Palacios IF, McAndrew T, Hueter I, Inglessis I, Baker JN, Kodali S, Leon MB, Svensson L, Pibarot P, Douglas PS, Fearon WF, Kirtane AJ, Maniar HS, Passeri JJ; PARTNER Investigators. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv. 2013;6:604-14.
4. Sannino A, Gargiulo G, Schiattarella GG, Brevetti L, Perrino C, Stabile E, Losi MA, Toscano E, Giugliano G, Scudiero F, Chiacchio E, Trimarco B, Esposito G. Increased mortality after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis and low ejection fraction: a meta-analysis of 6898 patients. Int J Cardiol. 2014;176:32-9.
5. Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Guéret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108: 319-24.
6. Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Gueret P, Tribouilloy C. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006;113:1738-44.
7. Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, Piazza N. Transcatheter aortic valve implantation in patients with LV dysfunction: impact on mortality and predictors of LV function recovery. J Invasive Cardiol. 2014;26:132-8.
8. Sato K, Kumar A, Jones BM, Mick SL, Krishnaswamy A, Grimm RA, Desai MY, Griffin BP, Rodriguez LL, Kapadia SR, Obuchowski NA, Popovic ZB. Reversibility of Cardiac Function Predicts Outcome After Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis. J Am Heart Assoc. 2017 Jul 11;6(7).
9. Freixa X, Chan J, Bonan R, Ibrahim R, Lamarche Y, Demers P, Basmadjian A, Ibrahim R, Cartier R, Asgar AW. Impact of coronary artery disease on left ventricular ejection fraction recovery following transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;85:450-8.
10. Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochellière R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928-36. 11. Hayek S, Pibarot P, Harzand A, Cheng JW, Gay H, Chrysohoou C, Ribeiro H, Rodes-Cabau J, Babaliaros V, Lerakis S. Dobutamine stress echocardiography for risk stratification of patients with low-gradient severe aortic stenosis undergoing TAVR. JACC Cardiovasc Imaging. 2015;8:380-2.
12. Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Munoz-Garcia AJ, Garcia Del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto WY, Clavel MA, de Agustin A, Serra V, Schindler JT, Dahou A, Puri R, Pelletier-Beaumont E, Cöté M, Pibarot P, Rodés-Cabau J. Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The TOPAS-TAVI Registry. J Am Coll Cardiol. 2018;71: 1297-308.
To download, please click below.