Introduction
The Asian population is growing and nearly four billion people live in Asia. Health care in Asia has been evolving rapidly over the last few decades and is influencing the life expectancy of its population. In fact, contemporary data indicate that the ageing population in Asia is growing as much as its economy (Figure 1),1. This suggests that aortic valve stenosis rates may also increase over time. Although transcatheter aortic valve implantation (TAVI) has been demonstrated clearly to be an effective treatment in severe aortic stenosis (AS) patients at intermediate to high risk for surgical aortic valve replacement (SAVR), uptake of the TAVI procedure in Asia compared with its population is low2,3,4. This therapy, however, is anticipated to grow substantially in the coming years. The Asian Pacific Society of Interventional Cardiology (APSIC) supported the establishment of this registry to chart prospectively the outcomes and uptake of TAVI in the Asia Pacific region.
Figure 1. World Health Chart.
Methods
The current Asia Pacific registry includes 14 centres and recruitment is ongoing. This current analysis includes data from seven sites in Hong Kong, Japan, Philippines, Singapore and Taiwan. The procedures were carried out between 2009 and 2017. Data were collected from each study site and then sent via web-based case report forms (CRF) to the National University Heart Centre Singapore (NUHCS) Cardiovascular Research Institute for collation and analysis. The ethics committees of the participating centres approved data collection. At some sites, patients’ informed consents were collected. Patient selection, procedure protocol and post-procedural care were performed according to local practice.
DATA AND DEFINITIONS
Preoperative baseline and outcome data were collected based on a uniform reporting template (CRF) and standardised definitions.
Postoperative outcomes were defined according to the updated Valve Academic Research Consortium (VARC-2) definitions5. The 30-day postoperative mortality is defined as all deaths that occurred within 30 days from the procedure.
The echocardiographic measurements were carried out according to the American Society of Echocardiography (ASE) guidelines6,7.
STATISTICAL ANALYSIS
Data are presented as frequency/percentages and mean±standard deviation, depending on the nature of the data. Confirmatory ana-ly-sis of 30-day mortality was performed with logistic regression in the context of a generalised structural equation model (gSEM). The advantage of using the gSEM framework was that both ana-ly-ses could be performed in one single framework. A backward elimination procedure (removal p>0.05) was also incorporated for model selection. Analyses were performed using Stata MP version 14 (StataCorp, College Station, TX, USA); all statistical tests were conducted at a 5% level of significance.
Results
STUDY POPULATION
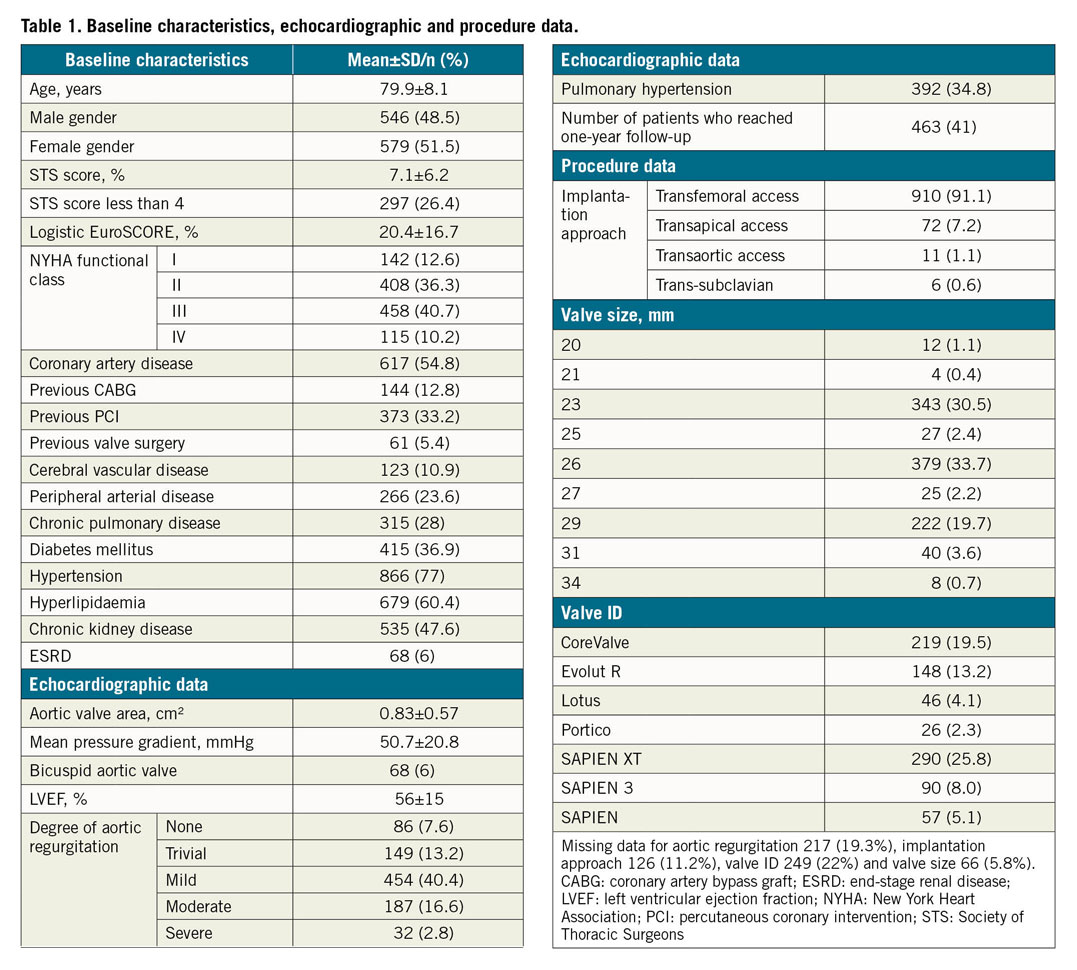
The study population comprises 1,125 patients enrolled from February 2009 to December 2017. Mean age was 79.9±8.1 years; 48.5% were male. Mean logistic EuroSCORE was 20.4±16.7; mean Society of Thoracic Surgeons (STS) score was 7.1±6.2. The New York Heart Association (NYHA) functional class was I (12.6%), II (36.3%), III (40.7%) and IV (10.2%). Preoperative clinical variables of the patients are listed in Table 1.
BASELINE ECHOCARDIOGRAPHIC PARAMETERS
All the patients underwent preoperative echocardiographic examination and baseline data were collected. The mean baseline transaortic gradient was 50.7±20.8 mmHg and the aortic valve area 0.83±0.57 cm2. There were 32 patients (2.8%) with concomitant severe aortic regurgitation and 68 patients (6%) had a bicuspid aortic valve. The mean left ventricular ejection fraction (LVEF) was 50.7±15% and a significant percentage (34.8%) had pulmonary hypertension based on echocardiographic measurement of pulmonary artery systolic pressure (PASP). Preoperative baseline echocardiographic data are listed in Table 1.
PROCEDURE DATA
The access routes included transfemoral, 910 (80.9%), transaortic, 11 (1%) and trans-subclavian, 6 (0.5%). The remaining 72 (6.4%) were performed via the transapical access. The most common valve platform used was the balloon-expandable valve and the most commonly used valve size was 26 mm. The post-procedure mean transaortic pressure gradient was 10.7±7.0 mmHg, while the mean post-procedure aortic valve area improved significantly (1.7±0.4 cm2). In this registry, 70.5% received first-generation TAVI devices and 29.5% received later-generation TAVI devices. The 30-day mortality rate of first-generation devices was 3.5% and that of later-generation devices was 1.1%.
THIRTY-DAY MORTALITY
All cause 30-day mortality was 2.5%. The causes of 30-day mortality are listed in Figure 2. The most common cause of death was cardiovascular (71.4%). The majority of the patients (68%) had a history of ischaemic heart disease, and 32% had a history of cerebrovascular accident. Based on the postoperative echocardiography, 17% of patients had moderate paravalvular regurgitation and 7% had mild paravalvular regurgitation. Based on the timeline, we also arbitrarily divided mortality outcomes into two groups, TAVI before 2014 and TAVI after 2014. The 30-day mortality before 2014 was 4.7% and it improved to 1.9% after 2014.
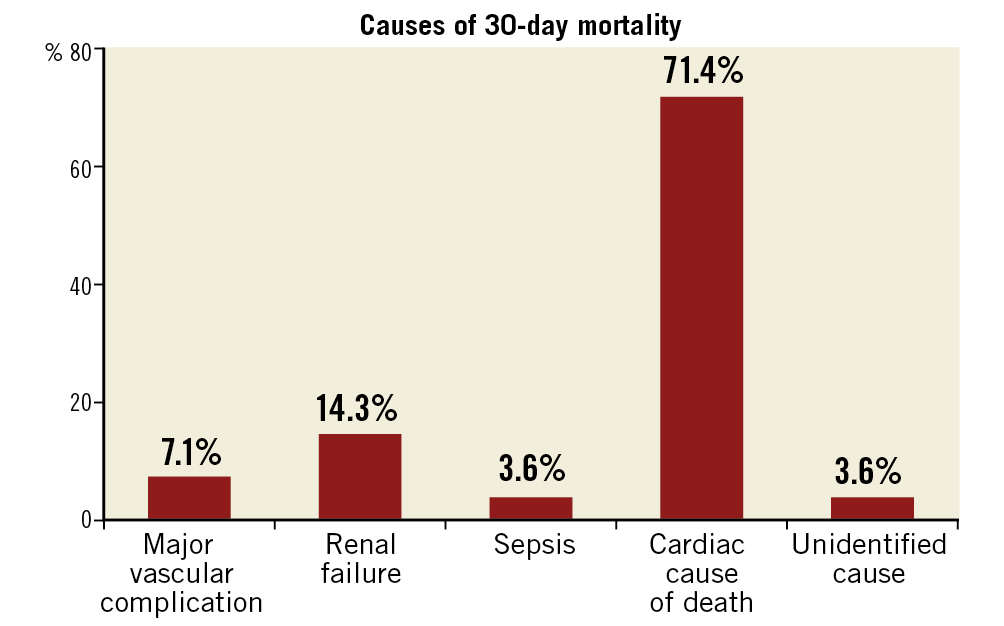
Figure 2. Causes of 30-day mortality.
ACUTE PROCEDURAL OUTCOME
The 30-day outcomes of acute kidney injury (AKI), stroke, major vascular complications and major bleeding, defined according to VARC-2, are presented in Figure 3. In this registry, AKI occurred in 77 patients (6.8%). In fact, AKI was the most common complication, followed by major vascular complications (5.8%, n=65). Stroke occurred in 14 patients (1.2%) and one-year mortality in stroke patients was high (35%, n=5). The permanent pacemaker (PPM) implantation rate at 30 days was 10.6% (n=119). Paravalvular regurgitation after TAVI was trivial in 135 patients (12.0%), mild in 184 (16.4%), moderate in 74 (6.6%) and severe in 1 patient (0.1%). There was 0.58%, 0.67% and 0.85% of coronary occlusion, aortic root rupture and emergency valve-in-valve, respectively. In this registry, the rate of major vascular complications from transfemoral access was 4.9% and from other access sites was 11.2%.
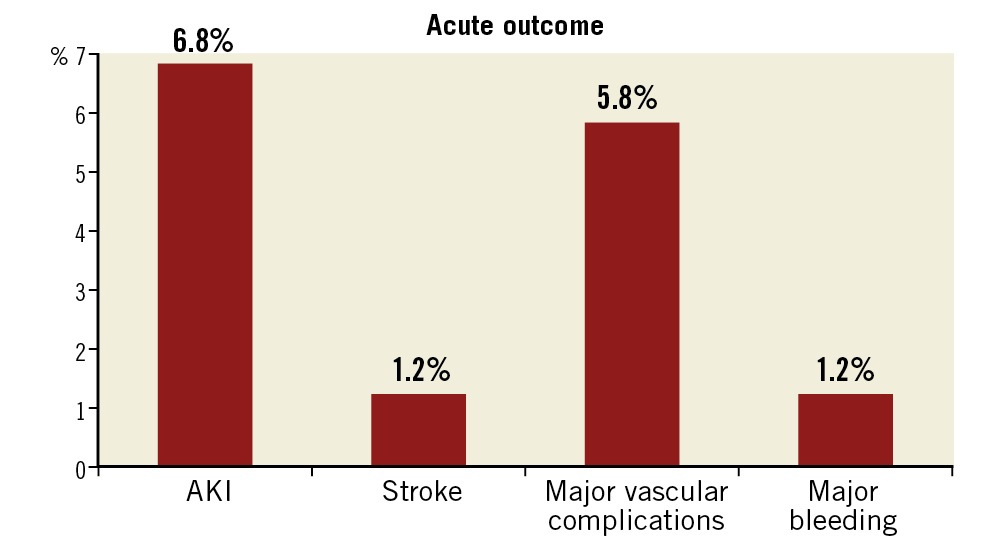
Figure 3. Acute outcomes of post-TAVI patients.
ONE-YEAR ALL-CAUSE MORTALITY
The one-year all-cause mortality rate among the post-TAVI patients was 8.8%.
PREDICTORS OF MORTALITY
Preprocedural predictors of 30-day all-cause mortality included risk scores such as the logistic EuroSCORE and the Society of Thoracic Surgeons (STS) score. A baseline logistic EuroSCORE of more than 16 was independently associated with a 2.8-times increased risk of 30-day all-cause mortality (p=0.016). Similarly, an STS score of more than 8 was independently associated with a 5.2-times increased risk of 30-day mortality (p<0.001).
The subsequent multivariate analyses were performed without these two composite risk scores in order to identify the specific independent risk factor(s) associated with 30-day mortality. Preprocedural and post-procedural cerebrovascular events were found to be significantly associated with 30-day mortality. Other things being equal, a patient who had suffered from a preprocedural cerebrovascular accident (CVA) could expect his or her odds of early death to be increased by 4.9 times (p=0.005). Similarly, a patient who had post-procedural CVA could expect the odds of early death to be increased by 9.2 times (p=0.008). However, preprocedural CVA was not significantly associated with the occurrence of post-procedural CVA (odds ratio [OR] 1.6, p=0.546). There were only two patients who suffered from preprocedural and post-procedural CVA.
Discussion
This is the first report of the APSIC-sponsored TAVI registry. In summary, we found that acute procedural success in this intermediate to high surgical risk cohort was good. There were reasonably low mortality rates and acceptable complication rates. Importantly, it demonstrated that both the logistic EuroSCORE and STS scores were independent predictors of mortality pre procedure, while cerebrovascular events were independent procedure-related predictors of morbidity and mortality. In this registry, the majority of the patients were female. Even though there have been concerns about the small annulus size in the Asian population, in this registry more than 65% of patients required valve sizes of 26 mm or larger. In this registry, the proportion of bicuspid aortic valve patients (6%) is lower compared to other Asian sites (e.g., China TAVI registries). This may be related to the average age of this cohort of patients (mean 79 years). It is speculated that the increased number of bicuspid valve patients in the Chinese cohorts may be due to the recruitment of younger patients in their study.
COMPARISON TO OTHER PUBLISHED REGISTRIES
This registry is supported and endorsed by the APSIC. This is an important publication which cements the role that APSIC plays in promoting collaborative research.
There are currently two published Asian registries. These are the Asian TAVI registry followed by the Japanese OCEAN-TAVI registry8,9. The unique difference is that this registry will continue to recruit patients from North and also South-East Asian patients. Also, this means that collectively it includes patients who are of more heterogenous ethnicity as well as incorporating varied implant volumes and experience. The early outcomes published in this study are not significantly different from the other two Asian studies thus far. In the patients recruited in this study, mostly second-generation TAVI devices (e.g., SAPIEN XT [Edwards Lifesciences, Irvine, CA, USA] and CoreValve® [Medtronic, Minneapolis, MN, USA]) were utilised, and in only a few patients were very early generation devices utilised. There were also some third-generation devices (Evolut™ R [Medtronic], SAPIEN 3 [Edwards Lifesciences], Lotus™ [Boston Scientific, Marlborough, MA, USA]) used. The patients included were mostly intermediate- to high-risk patients. The low 30-day mortality rates should therefore be compared to more contemporary studies utilising the appropriate generation of devices. Figure 4 shows that the outcomes compare favourably to other studies with relatively similar baseline risk profiles9,10,11,12,13.
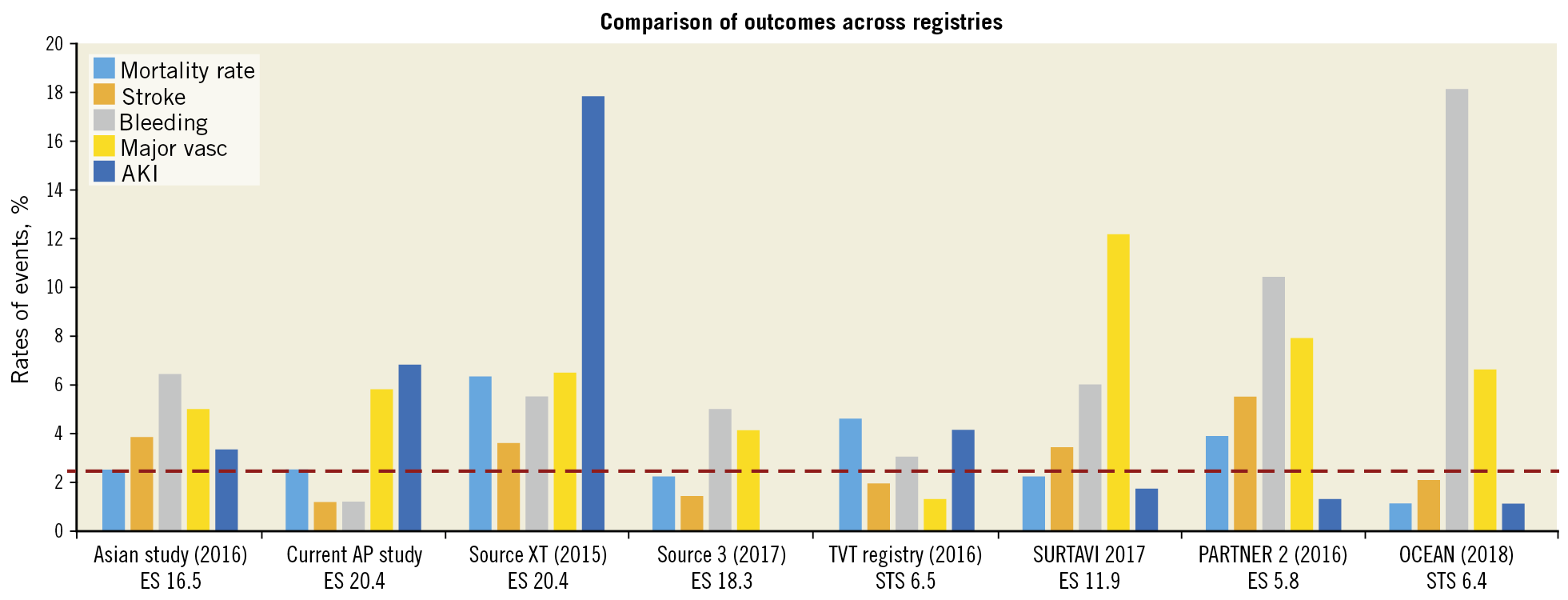
Figure 4. Comparison of outcomes across registries13.
ROLE OF THE LOGISTIC EUROSCORE OR STS SCORE
There are currently several risk scores being utilised for patients undergoing TAVI. These include the STS/American College of Cardiology (ACC) transcatheter valve therapy (TVT) mortality risk score, EuroSCORE I, EuroSCORE II, STS PROM and German AV score14. These scores serve several purposes, two of which are especially important. The first is to predict the 30-day mortality of patients undergoing surgery or TAVI and the second is the ability to compare across other studies. In this study, the traditional logistic EuroSCORE as well as the STS score were utilised to enable comparison across registries. While the logistic EuroSCORE and EuroSCORE II have been validated, with the latter being superior in Chinese patients for valve surgery or coronary artery bypass graft (CABG) surgery, these have not been validated in TAVI patients. This study showed that a logistic EuroSCORE of >16 and STS score >8 could predict mortality in this group of Asian TAVI patients. In the absence of other validated scoring systems, this may be utilised as a preprocedural predictor for contemporary practice.
IMPACT OF STROKE
Stroke in this study occurred infrequently (1.2%). It is important to point out that this refers to clinically evident stroke and site-reported stroke. Thus, the true incidence may be higher. It is well established that systematic neurological evaluation by trained neurologists may detect a higher incidence of stroke post TAVI. Despite the low incidence of stroke, the impact of stroke on 30-day mortality is striking. Prior studies have also demonstrated that stroke after TAVI can be devastating. A German study showed a fivefold increase in the 30-day mortality after post-TAVI stroke15. These results mandate that the Asia Pacific TAVI community should focus future attention on this complication. This would require a more in-depth assessment of risks for stroke and assessment of the impact of cerebral protection devices in the Asia Pacific cohort of patients.
Limitations
Although standardised reporting was encouraged, techniques and choices of valve were left to the discretion of individual sites. The clinical outcomes were not independently adjudicated, and echocardiographic reporting was not evaluated by a core laboratory. This may have introduced bias into the study. However, the hard outcome of mortality is reasonably robust given that the current sites have a systematic mechanism for 30-day follow-up.
Conclusions
This initial report of the APSIC-supported Asia Pacific TAVI registry demonstrates that the 30-day outcomes were good. A high logistic EuroSCORE and high STS scores pre procedure, as well as post-procedure stroke are associated with increased 30-day mortality.
Impact on daily practiceOne of the important messages from this registry is the stroke risk in post-TAVI patients which can have a serious impact on 30-day mortality and morbidity. For the Asian population, stroke risk assessment before the procedure is crucially important based on this early report of the Asia Pacific TAVI registry. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

