Andie H. Djohan1*, MBBS; Ching-Hui Sia2, MBBS; Joshua Ping-Yun Loh2,3, MBBS
AsiaIntervention 2019;5:32-40, DOI: 10.4244/AIJ-D-18-00015
1. Department of Medicine, National University Health System, Singapore; 2. Department of Cardiology, National University Heart Centre Singapore, National University Health System, Singapore; 3. Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Abstract
Concomitant chronic kidney disease (CKD) and coronary artery disease (CAD) is known to have poor outcomes. With a thorough literature review, we discuss the pathophysiological basis behind accelerated atherosclerosis in CKD, and the role of percutaneous coronary intervention (PCI) in these patients, focusing on drug-eluting stents, coronary artery bypass grafting, and adverse outcomes. We discuss factors contributing to poor outcomes in these patients, and the need for more work in this subgroup.
Abbreviations
BMS: bare metal stent(s)
CABG: coronary artery bypass grafting
CAC: coronary artery calcification
CAD: coronary artery disease
CHF: congestive heart failure
CKD: chronic kidney disease
DAPT: dual antiplatelet therapy
DES: drug-eluting stent(s)
EES: everolimus-eluting stent(s)
eGFR: estimated glomerular filtration rate
ESRD: end-stage renal disease
LVD: left ventricular dysfunction
MI: myocardial infarction
OAS: orbital atherectomy system
PCI: percutaneous coronary intervention
RA: rotational atherectomy
SES: sirolimus-eluting stent(s)
ST: stent thrombosis
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Chronic kidney disease (CKD) is defined as the presence of kidney damage or reduced kidney function (eGFR <60 mL/min/1.73 m2) for ≥3 months1. Most studies concluded that an eGFR <60 mL/min/1.73 m2 is associated with increased risk of restenosis, recurrent myocardial infarction (MI), congestive heart failure (CHF) and mortality2. The current recommendation for DES use in end-stage renal disease (ESRD) patients is deduced from extrapolation of information from patients with normal renal function3. Furthermore, CKD is sub-classified into stages 1-51, each associated with different mortality and revascularisation events with best pharmacological therapy, PCI, and coronary artery bypass grafting (CABG).
Unique vascular pathobiology in CKD
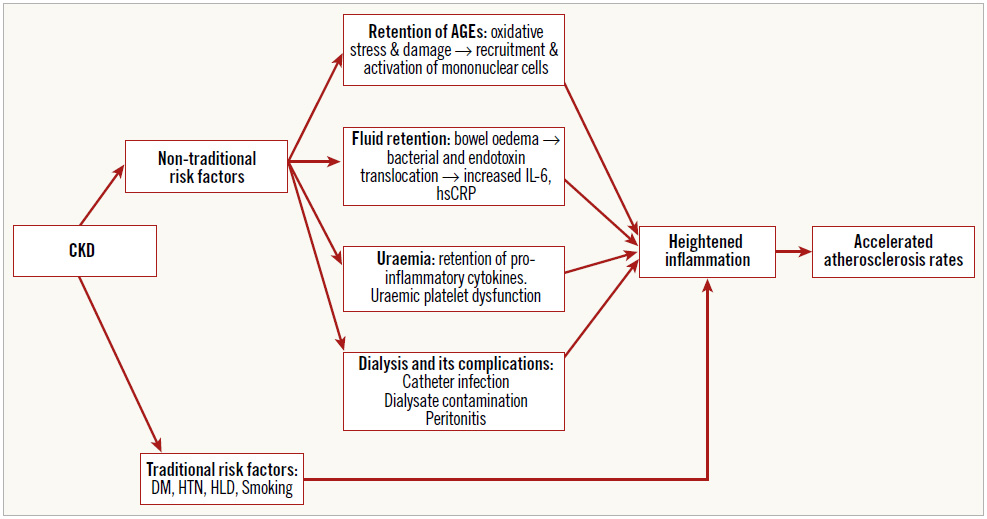
Inflammation drives atherosclerosis4. CKD patients have co-existing traditional cardiovascular risk factors propagating inflammation. Among non-traditional risk factors, contributors to inflammation include advanced glycated end products (AGEs), uraemia, peritoneal dialysis and haemodialysis5.
Retention of AGEs secondary to decreased renal function causes oxidative damage, recruitment of mononuclear cells and an inflammation, which is intensified by fluid retention via bacterial or endotoxin translocation from bowel oedema, producing pro-inflammatory cytokines (interleukin-6 [IL-6], high-sensitivity CRP [hsCRP]) (Figure 1). These are not adequately cleared secondary to uraemia. This is of clinical importance as IL-6 and hsCRP are independent predictors of mortality in CKD6.
Reduced renal function is associated with disruption of the balance between endothelin and nitric oxide, functional platelet abnormalities and coagulopathy7, predisposing to atherosclerosis.
Efficacy and safety of DES compared to BMS/CABG
The bare metal stent (BMS) superseded balloon angioplasty as the treatment of choice following improved angiographic and clinical outcomes. However, BMS-related adverse events such as in-stent restenosis with rates of 20-30%8 led to the development of the drug-eluting stent (DES) which shares the same complications but at a delayed interval.
New-generation DES with a reduced load of antiproliferative drugs, thinner metallic struts and improved biocompatibility of stent polymer are the new standard of care9. The NORSTENT trial10 failed to demonstrate benefits in mortality and non-fatal MI with the use of DES over BMS but revealed benefits in stent thrombosis and repeat revascularisation. Furthermore, NORSTENT did not target patients with CKD where the improved designs of newgeneration DES might be less thrombogenic. Also, a recent metaanalysis comparing DES and BMS use in CKD patients concluded with observed benefits seen across mortality, MI, stent thrombosis (ST) and target vessel revascularisation (TVR). No difference was observed between first- and second-generation DES11. A meta-analysis comparing second-generation DES (everolimus- eluting stent [EES]) with CABG reported increased rates of MI and repeat revascularisation in patients receiving EES, despite comparable mortality rates12. Hence, there might be a role for consideration of CABG in patients who are surgically fit to improve their quality of life.
Figure 1. Pathophysiology of accelerated atherosclerosis in CKD. AGEs: advanced glycated end products; CKD: chronic kidney disease; DM: diabetes mellitus; HLD: hyperlipidaemia; hsCRP: high-sensitivity C-reactive protein; HTN: hypertension; IL-6: interleukin-6
Comparative outcomes of DES versus BMS in CKD
Mortality rates are inversely related to the degree of renal dysfunction, with tripling of mortality rates in patients with both CAD and severe CKD compared to patients with normal renal function13.
MORTALITY
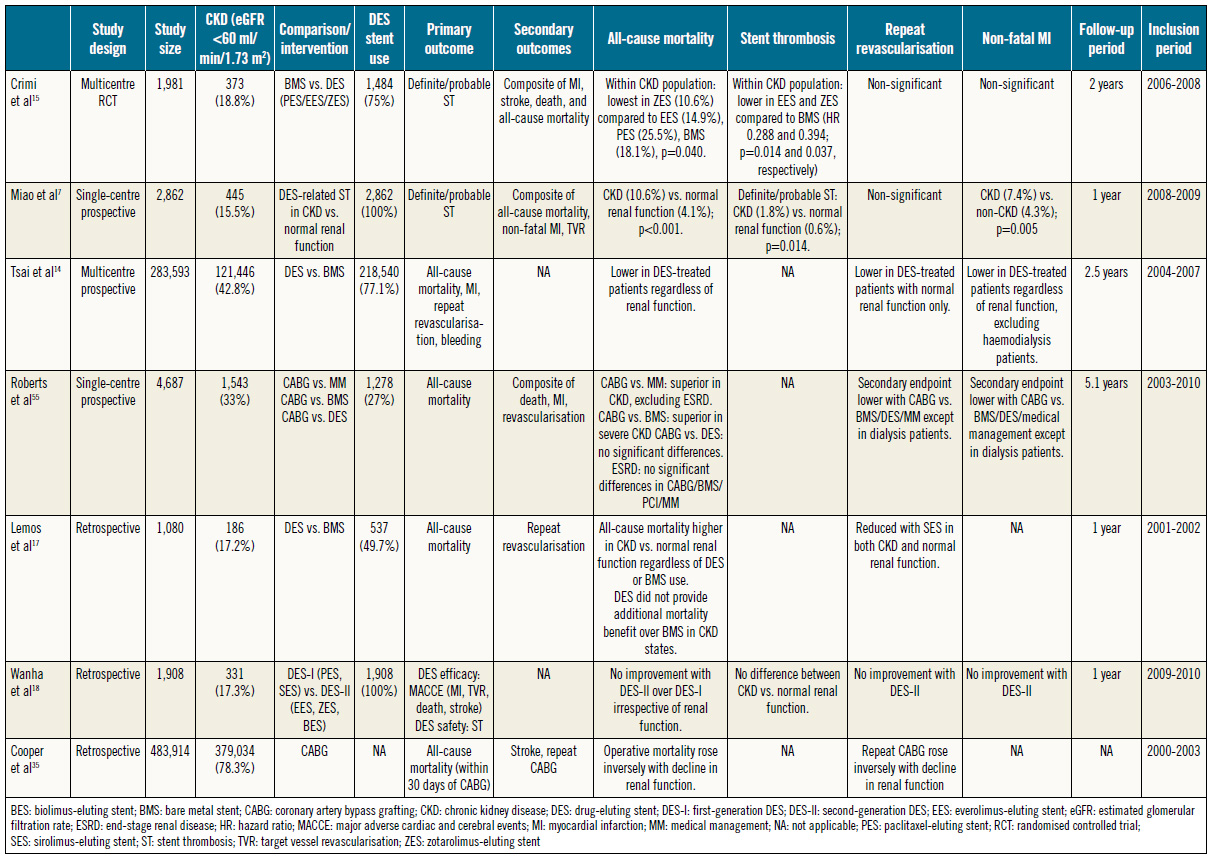
Tsai et al14 (Table 1) reported benefits in all-cause mortality with DES compared to BMS both in patients with normal renal function and in those with CKD. This was echoed by similar findings in a post hoc analysis of the PRODIGY trial15 and in a study by Jeong et al16. However, Lemos et al17 reported insignificant differences in mortality despite improvements in clinical restenosis.
The benefits of second- over first-generation DES in the CKD population are unclear, with a retrospective analysis failing to demonstrate mortality benefits with the use of second-generation DES18, probably due to systemic factors which are recognised to worsen oxidative stress and systemic inflammation independently19. Other contributing factors include left ventricular dysfunction (LVD) which is associated with adverse outcomes post PCI20.
STENT THROMBOSIS
An increased risk of stent thrombosis secondary to abnormal vascular pathobiology in CKD was demonstrated by a study which found that rates of ST were significantly raised in CKD compared to normal renal function at one-year follow-up post DES implantation7.
In PRODIGY, the number needed to treat to prevent one definite or probable ST at two-year follow-up was 20 in CKD patients versus 50 in patients with normal renal function, reinforcing the significant benefits of DES15.
The higher incidence of ST in CKD is related to increased severity of systemic atherosclerosis, and diffuse and calcified coronary artery disease which increases the risk of stent malapposition and underexpansion7. Restenosis rates following PCI range from 60-81% when assessed via repeat coronary angiography. In contrast to patients with normal kidneys, clinical restenosis is not raised in patients with CKD, suggesting silent progression of cardiac ischaemia, hence a high risk of adverse cardiac events21.
TARGET VESSEL/LESION REVASCULARISATION
The benefits of DES over BMS in relation to TVR/target lesion revascularisation (TLR) are unclear. While several studies have demonstrated a reduced incidence of repeat revascularisation with DES compared to BMS15,22, Tsai et al’s14 work on DES implantation demonstrated a significant reduction in repeat revascularisation only in patients with normal renal function. Besides inflammation, alternative explanations include antiplatelet resistance observed in chronic renal failure23,24. Nevertheless, a lack of guideline-directed antiplatelet therapy in the CKD population might be contributory2,25,26, which needs to be explored further.
NON-FATAL MI
The incidence of higher MI rates post PCI is universally increased in CKD patients compared to those with normal renal function7,27. Despite an overall raised incidence of post-PCI MI in CKD patients, the use of DES over BMS is associated with reduced MI rates14. Increased MI rates in CKD patients despite DES use indicate that systemic inflammation and/or metabolic derangement have a greater impact on endpoints. This is insufficiently addressed by the local effects of antiproliferatives in current DES27.
In summary, the current evidence suggests that CKD is an independent predictor of mortality, MI and stent thrombosis. Also, DES are superior to BMS in the CKD population, with the caveat that the requirement for dual antiplatelet therapy (DAPT) is unlikely to disrupt subsequent non-cardiac treatment.
Revascularisation in ESRD/haemodialysis
DES VS. BMS
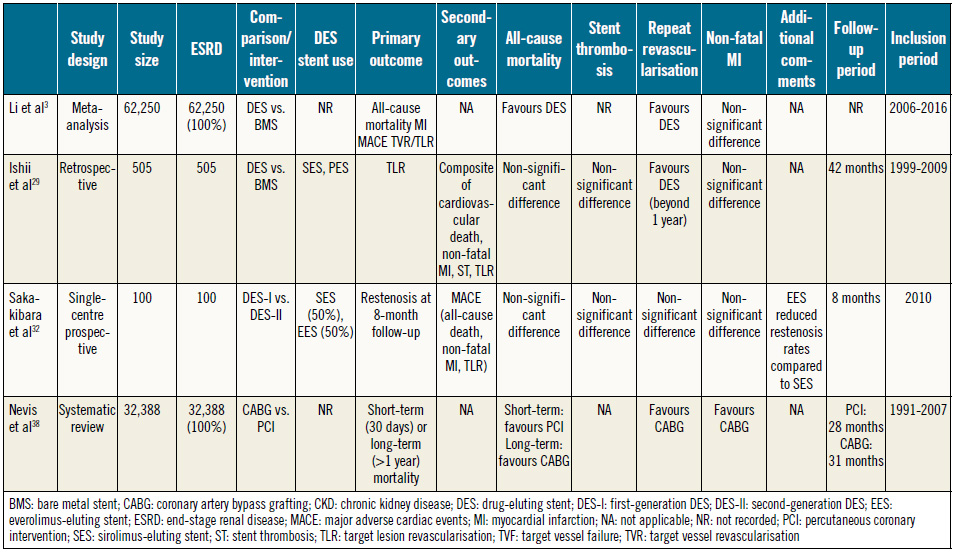
In line with contemporary guidelines advocating the use of DES in ESRD patients on dialysis28, a meta-analysis has demonstrated mortality and adverse cardiac event benefits in ESRD patients who are treated with DES3.
Despite a lack of benefit at one-year follow-up, Ishii et al29 (Table 2) reported reduced revascularisation rates in haemodialysis patients treated with DES over BMS on longer follow-up29,30. This suggests the need for adequate endothelialisation of the deployed stent in the uraemic state, hence the need for extended follow-up.
FIRST- VS. SECOND-GENERATION DES
Despite a paucity of data on outcomes of second-generation DES in patients on maintenance haemodialysis, ESRD and haemodialysis are recognised as major predictors of adverse outcome following first-generation DES implantation, with approximately double the incidence of target vessel failure (TVF) in patients who received sirolimus-eluting stents (SES) compared to non-haemodialysis patients31.
Table 1. Summary of studies comparing coronary revascularisation approaches in CKD (including ESRD).
Table 2. Summary of studies comparing coronary revascularisation approaches in ESRD (dialysis-dependent).
When implemented on maintenance haemodialysis patients, EES significantly reduced the incidence of restenosis compared to SES with an equal safety profile32, with consistent results in improvements in diameter stenosis on follow-up compared with the OUCHPRO registry31. Mechanisms for reduction in restenosis rates include reduced arterial injury and inflammation secondary to thinner struts and polymer of second-generation DES, respectively32,33.
PCI VS. CABG
Despite increased early post-CABG mortality in ESRD34,35, Zheng et al30 demonstrated significant long-term benefits in mortality, MI, and repeat revascularisation compared to PCI. Supporting this is a systematic review reporting benefits in repeat revascularisation and major adverse cardiac events (MACE) with CABG, despite a higher early mortality risk36. The proposed pathophysiology includes significant medial calcification in haemodialysis patients, predisposing to stent underexpansion, reduced efficacy of eluted drugs and suboptimal endothelialisation of stent struts, culminating in restenosis and stent thrombosis37.
Nevertheless, there is an elevated baseline risk of long-term mortality in haemodialysis patients regardless of the choice of revascularisation technique38.
Rotational atherectomy (RA) for the treatment of coronary artery calcification (CAC)
Severely calcified coronary lesions have lower PCI success rates, higher complication rates, and suboptimal long-term results. Contemporary PCI guidelines recommend RA as an option for heavily calcified lesions that might not be adequately traversed or dilated prior to stent implantation39.
The ROTAXUS trial, comparing RA followed by stenting or stenting alone in complex native CAD, demonstrated higher success with use of RA and higher acute lumen gain post PCI. However, in-stent late lumen loss was significantly higher in the RA group compared to DES alone40, indicating that rotablation alone failed to increase the efficacy of DES.
The Diamondback 360® coronary orbital atherectomy system (OAS; Cardiovascular Systems, Inc., St. Paul, MN, USA) presents an alternative in revascularisation of CACs. ORBIT I demonstrated 98% device success (≤50% residual stenosis post-OAS treatment) while ORBIT II exceeded primary safety (freedom from MACE at 30 days) and efficacy (residual stenosis <50% post stent without inhospital major cardiac events) endpoints, highlighting its suitability for implementation in CACs. Furthermore, subgroup analysis of DES use revealed a lower rate of TLR compared to BMS41,42.
Besides technique, the approach to complex CACs should include accurate lesion assessment and characterisation which is poorly delineated by angiography alone41,43. Optical coherence tomography might be the supplemental imaging modality of choice for the assessment of intraluminal calcium thickness41. Accurate assessment will facilitate optimal stent placement, reduce stent underexpansion, malapposition, damage to the DES polymer coating and subsequent drug delivery42. Compared to bail-out atherectomy, planned atherectomy is associated with a reduced procedural time, less use of contrast and reduced rates of complications44.
Overall, our review suggests that, while results regarding mortality benefit are mixed when DES are compared with BMS in both CKD and ESRD, DES are shown to improve rates of MI and repeat revascularisation3,14,15,29. There is no significant benefit of second- over first-generation DES18. The benefits of rotational atherectomy warrant consideration for planned instead of bail-out use in appropriate lesions44.
Choice and duration of DAPT in CKD
DAPT use post PCI is critical to minimise the rate of adverse cardiovascular events25. Contemporary European and US guidelines recommend a DAPT duration of six to 12 months post DES deployment, followed by lifelong aspirin9,39. However, no consensus for DAPT drugs and duration in patients with CKD/ESRD exists, owing to the lack of clinical trials25. Though not targeted at CKD patients, the DAPT trial demonstrated that prolonged DAPT (30 months) significantly decreased rates of ST and the composite outcome of death, MI and stroke, at the expense of bleeding45. However, the extrapolation of findings from a non-CKD/ESRD population into this high-risk population is probably inappropriate, due to an increased incidence of cardiovascular events or bleeding complications after PCI in haemodialysis patients46,47.
Both CKD and prolonged DAPT independently predict elevated bleeding complications. However, it is uncertain whether prolonged DAPT worsens bleeding risk in CKD patients48.
A pooled analysis comparing the safety and efficacy of shortterm (three to six months) versus long-term (≥12 months) DAPT post DES implantation in CKD patients found that the presence and degree of CKD have no effect on the rates of coronary thrombotic events, regardless of the duration of DAPT25, which is in sync with a study by Baber et al48, where severity of CKD has no effect on cardiovascular risk post DAPT cessation. Further, Chen et al’s analysis49 in the haemodialysis subgroup reported a six-month DAPT duration cut-off that reduces post-PCI death or MI, but shows no difference in long-term outcomes when compared to longer duration of DAPT (>6 months). However, these studies used clopidogrel instead of more potent P2Y12 inhibitors such as prasugrel or ticagrelor, which may have had an impact on overall study outcome.
In summary, CKD and DAPT independently predict bleeding risk, while CKD contributes to an increased risk of ST. A lack of consensus regarding antiplatelet therapy in CKD/ESRD leads to a reduced use due to a perceived lack of benefit, coupled with fear of coagulopathy and antiplatelet resistance7,23,24. Also, our review suggests that thrombotic complications post cessation of DAPT are independent of the severity of CKD. Future studies concerning DAPT and bleeding complications ought to have a uniform use of antiplatelets and duration to minimise possible confounding effects on stent choice and CKD severity.
Role of CABG in CKD
The 2014 European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) Guidelines50 on myocardial revascularisation recommend CABG over PCI in patients with moderate to severe CKD and multivessel CAD, considering acceptable surgical risks and life expectancy beyond one year.
Given complex coronary lesions in CKD, findings from ASCERT reporting long-term mortality benefits with CABG over PCI in multivessel CAD could be applied. Though patients with CKD often have more complex coronary lesions with multivessel disease, increased coronary calcification, and the presence of thrombus in culprit coronary lesions, their SYNTAX score is comparable to patients with normal kidneys18. CABG should be considered since extensive coronary calcifications reduce PCI success rates and is also associated with significant improvements in symptoms and mortality51. The FREEDOM trial52 comparing coronary revascularisation techniques in CKD concluded that CKD is an independent risk factor for adverse events regardless of revascularisation strategy, where there is no evidence of additional benefit in outcomes according to CKD severity. Being an independent risk factor for stent thrombosis, coronary revascularisation in CKD has shifted in favour of CABG especially on long-term follow-up52,53. Nevertheless, CABG is superior to PCI with regard to reductions in rates of MI and repeat revascularisation regardless of renal function provided patients do not present with acute coronary syndrome52,54.
Comparing rates of adverse events in CABG against BMS in CKD/ESRD, CABG is associated with reduced mortality rates though there was statistical significance only in severe CKD (eGFR <30 mL/min/1.73 m2)55. A subsequent analysis comparing CABG against DES observed a trend towards mortality reduction for CABG without statistical significance. No mortality difference was observed in patients on dialysis, regardless of revascularisation strategy55.
However, a five-year follow-up on the SYNTAX trial demonstrated that a statistically significant long-term benefit in mortality, MI and stroke is associated with revascularisation with CABG over DES. Differences in event rates were attributed to the higher rates of all-cause death and repeat revascularisation in the CKD population who received DES, secondary to diffuse atherosclerosis and reduced prevalence of guideline-directed antiplatelet therapy26,52.
In conclusion, CKD and ESRD are independent risk factors for adverse events regardless of revascularisation strategy17,35. CABG is associated with long-term benefits in both mortality and adverse cardiac events both in patients with CKD and in those with ESRD38,55. However, physicians and patients ought to consider and accept higher risks of early complications such as mortality and stroke53, stressing the importance of patient selection.
Conclusions
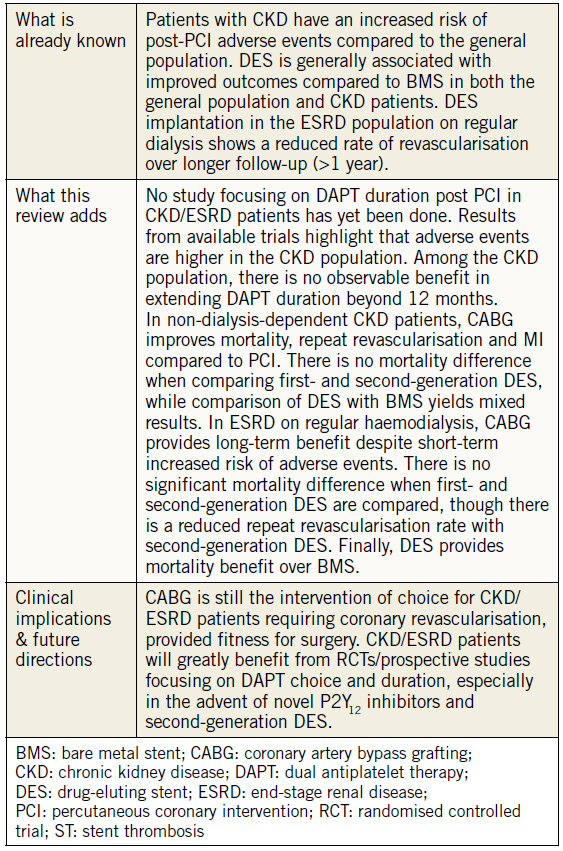
Despite an increasing prevalence of patients with CKD and CAD, there remain limited studies evaluating the optimum method of coronary revascularisation, and DAPT duration in this subgroup. Our review highlights the following. 1) CKD is an independent predictor of mortality, MI and stent thrombosis. 2) DES is superior to BMS in a CKD population. 3) CKD and DAPT are independent predictors of bleeding complications post PCI, though the severity of CKD seems not to affect the rates of coronary thrombotic events. 4) CABG is the revascularisation modality of choice (Table 3) in CKD patients who are surgically fit due to mortality and symptomatic benefits, and the reduced need for repeat revascularisation compared to PCI. 5) It is also imperative for a consensus on DAPT choice and duration to be validated to maximise the benefits of high-risk, invasive procedures in this fragile subset of patients
Table 3. Summary of findings and clinical implications.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. [No authors listed]. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19-62.
2. McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725-8.
3. Li S, Ye D, Chen G, Xu W. Meta-Analysis of Comparison of Drug-Eluting Stents and Bare-Metal Stents in Patients on Dialysis. Am J Cardiol. 2017;119:1186-92.
4. Crea F, Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation. 2017;136:1155-66.
5. Yao Q, Axelsson J, Stenvinkel P, Lindholm B. Chronic systemic inflammation in dialysis patients: an update on causes and consequences. ASAIO J. 2004;50:Iii-Ivii.
6. Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End- Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib Nephrol. 2017;191:32-43.
7. Miao Y, Yu-Jie Z, Zhi-Jian W, Dong-Mei S, Yu-Yang L, Ying- Xin Z, Fei G, Shi-Wei Y, De-An J. Chronic kidney disease and the risk of stent thrombosis after percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv. 2012;80:361-7.
8. Buchanan K, Steinvil A, Waksman R. Does the new generation of drug-eluting stents render bare metal stents obsolete? Cardiovasc Revasc Med. 2017;18:456-61.
9. Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head S, Jüni S, Kappetein A, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimgli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-619.
10. Bonaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygard O, Nilsen DW, Klow NE, Uchto M, Trovik T, Bendz B, Stavnes S, Bjornerheim R, Larsen AI, Slette M, Steigen T, Jakobsen OJ, Bleie O, Fossum E, Hanssen TA, Dahl-Eriksen O, Njolstad I, Rasmussen K, Wilsgaard T, Nordrehaug JE; NORSTENT Investigators. Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease. N Engl J Med. 2016;375:1242-52.
11. Volodarskiy A, Kumar S, Pracon R, Sidhu M, Kretov E, Mazurek T, Bockeria O, Kaul U, Bangalore S. Drug-Eluting vs Bare-Metal Stents in Patients With Chronic Kidney Disease and Coronary Artery Disease: Insights From a Systematic Review and Meta-Analysis. J Invasive Cardiol. 2018;30:10-17.
12. Bundhun PK, Pursun M, Teeluck AR, Bhurtu A, Soogund MZ, Huang WQ. Adverse Cardiovascular Outcomes associated with Coronary Artery Bypass Surgery and Percutaneous Coronary Intervention with Everolimus Eluting Stents: A Meta-Analysis. Sci Rep. 2016;6:35869.
13. van Domburg RT, Hoeks SE, Welten GM, Chonchol M, Elhendy A, Poldermans D. Renal insufficiency and mortality in patients with known or suspected coronary artery disease. J Am Soc Nephrol. 2008;19:158-63.
14. Tsai TT, Messenger JC, Brennan JM, Patel UD, Dai D, Piana RN, Anstrom KJ, Eisenstein EL, Dokholyan RS, Peterson ED, Douglas PS. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. 2011;58:1859-69.
15. Crimi G, Leonardi S, Costa F, Adamo M, Ariotti S, Valgimigli M. Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifiable choice? A post-hoc analysis of the all comer PRODIGY trial. Int J Cardiol. 2016;212:110-7.
16. Jeong YH, Hong MK, Lee CW, Park DW, Kim YH, Kim JJ, Park SW, Park SJ. Impact of significant chronic kidney disease on long-term clinical outcomes after drug-eluting stent versus bare metal stent implantation. Int J Cardiol. 2008;125:36-40.
17. Lemos PA, Arampatzis CA, Hoye A, Daemen J, Ong AT, Saia F, van der Giessen WJ, McFadden EP, Sianos G, Smits PC, de Feyter P, Hofma SH, van Domburg RT, Serruys PW. Impact of baseline renal function on mortality after percutaneous coronary intervention with sirolimus-eluting stents or bare metal stents. Am J Cardiol. 2005;95:167-72.
18. Wanha W, Kawecki D, Roleder T, Pluta A, Marcinkiewicz K, Morawiec B, Dola J, Gladysz S, Pawlowski T, Smolka G, Ochala A, Nowalany-Kozielska E, Wojakowski W. Long-Term Percutaneous Coronary Intervention Outcomes of Patients with Chronic Kidney Disease in the Era of Second-Generation Drug-Eluting Stents. Cardiorenal Med. 2017;7:85-95.
19. Landes U, Kornowski R, Assali A, Vaknin-Assa H, Greenberg G, Lev EI, Bental T. Predictors of long term outcomes in 11,441 consecutive patients following percutaneous coronary interventions. Am J Cardiol. 2015;115:855-9.
20. De Silva K, Webb I, Sicard P, Lockie T, Pattinson S, Redwood S, Perera D. Does left ventricular function continue to influence mortality following contemporary percutaneous coronary intervention? Coron Artery Dis. 2012;23:155-61.
21. Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113-9.
22. Halkin A, Mehran R, Casey CW, Gordon P, Matthews R, Wilson BH, Leon MB, Russell ME, Ellis SG, Stone GW. Impact of moderate renal insufficiency on restenosis and adverse clinical events after paclitaxel-eluting and bare metal stent implantation: results from the TAXUS-IV Trial. Am Heart J. 2005;150:1163-70.
23. Lee K, Lee S, Lee JW, Kim SY, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH. The significance of clopidogrel lowresponsiveness on stent thrombosis and cardiac death assessed by the verifynow p(2)y(12) assay in patients with acute coronary syndrome within 6 months after drug-eluting stent implantation. Korean Circ J. 2009;39:512-8.
24. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505-16.
25. Hwang D, Park KW, Lee JM, Rhee TM, Hong MK, Jang Y, Valgimigli M, Colombo A, Gilard M, Palmerini T, Stone GW, Kim HS. Efficacy and safety of dual antiplatelet therapy after coronary stenting in patients with chronic kidney disease. Am Heart J. 2018;197:103-12.
26. Milojevic M, Head SJ, Mack MJ, Mohr FW, Morice MC, Dawkins KD, Holmes DR Jr, Serruys PW, Kappetein AP. The impact of chronic kidney disease on outcomes following percutaneous coronary intervention versus coronary artery bypass grafting in patients with complex coronary artery disease: five-year follow-up of the SYNTAX trial. EuroIntervention. 2018;14:102-11.
27. Choi DH, Park KW, Yang HM, Lee HY, Park JS, Kang HJ, Kim YJ, Koo BK, Oh BH, Park YB, Kim HS. Renal dysfunction and high levels of hsCRP are additively associated with hard endpoints after percutaneous coronary intervention with drug eluting stents. Int J Cardiol. 2011;149:174-81.
28. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S31-153.
29. Ishii H, Toriyama T, Aoyama T, Takahashi H, Tanaka M, Yoshikawa D, Hayashi M, Yasuda Y, Maruyama S, Matsuo S, Matsubara T, Murohara T. Percutaneous coronary intervention with bare metal stent vs. drug-eluting stent in hemodialysis patients. Circ J. 2012;76:1609-15.
30. Zheng H, Xue S, Lian F, Huang RT, Hu ZL, Wang YY. Metaanalysis of clinical studies comparing coronary artery bypass grafting with percutaneous coronary intervention in patients with end-stage renal disease. Eur J Cardiothorac Surg. 2013;43:459-67.
31. Ikari Y, Kyono H, Isshiki T, Ishizuka S, Nasu K, Sano K, Okada H, Sugano T, Uehara Y. Usefulness of Everolimus-Eluting Coronary Stent Implantation in Patients on Maintenance Hemodialysis. Am J Cardiol. 2015;116:872-6.
32. Sakakibara T, Ishii H, Toriyama T, Aoyama T, Takahashi H, Kamoi D, Kawamura Y, Kawashima K, Yoneda K, Amano T, Tanaka M, Yoshikawa D, Hayashi M, Matsubara T, Murohara T. Sirolimus-eluting stent vs. everolimus-eluting stent for coronary intervention in patients on chronic hemodialysis. Circ J. 2012;76:351-5.
33. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816-21.
34. Liu JY, Birkmeyer NJ, Sanders JH, Morton JR, Henriques HF, Lahey SJ, Dow RW, Maloney C, DiScipio AW, Clough R, Leavitt BJ, O’Connor GT. Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 2000;102:2973-7.
35. Cooper WA, O’Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063-70.
36. Deo SV, Shah IK, Dunlay SM, Erwin PJ, Dillon JM, Park SJ. Myocardial revascularisation in renal dysfunction: a systematic review and meta-analysis. Heart Lung Circ. 2013;22:827-35.
37. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703-14.
38. Nevis IF, Mathew A, Novick RJ, Parikh CR, Devereaux PJ, Natarajan MK, Iansavichus AV, Cuerden MS, Garg AX. Optimal method of coronary revascularization in patients receiving dialysis: systematic review. Clin J Am Soc Nephrol. 2009;4:369-78.
39. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH; American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44-122.
40. Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, Schofer J, King L, Neumann FJ, Khattab AA. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-9.
41. Shlofmitz E, Martinsen BJ, Lee M, Rao SV, Généreux P, Higgins J, Chambers JW, Kirtane AJ, Brilakis ES, Kandzari DE, Sharma SK, Shlofmitz R. Orbital atherectomy for the treatment of severely calcified coronary lesions: evidence, technique, and best practices. Expert Rev Med Devices. 2017;14:867-79.
42. Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, Shlofmitz RA, Dulas DD, Arab D, Khanna PK, Lee AC, Ghali MG, Shah RR, Davis TP, Kim CY, Tai Z, Patel KC, Puma JA, Makam P, Bertolet BD, Nseir GY. Pivotal Trial to Evaluate the Safety and Efficacy of the Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-8.
43. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ, Leon MB. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959-65.
44. Allali A, Abdel-Wahab M, Sulimov DS, Jose J, Geist V, Kassner G, Richardt G, Toelg R. Comparison of Bailout and Planned Rotational Atherectomy for Heavily Calcified Coronary Lesions: A Single-Center Experience. J Interv Cardiol. 2017;30: 124-33.
45. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155-66.
46. Mulukutla SR, Marroquin OC, Vlachos HA, Selzer F, Toma C, Kip KE, Abbott JD, Holper E, Lee JS, Khandhar S, Kutcher M, Kelsey S, Smith C, Faxon D, Williams DO. Benefit of long-term dual anti-platelet therapy in patients treated with drugeluting stents: from the NHLBI dynamic registry. Am J Cardiol. 2013;111:486-92.
47. Hiremath S, Holden RM, Fergusson D, Zimmerman DL. Antiplatelet medications in hemodialysis patients: a systematic review of bleeding rates. Clin J Am Soc Nephrol. 2009;4:1347-55.
48. Baber U, Li SX, Pinnelas R, Pocock SJ, Krucoff MW, Ariti C, Gibson CM, Steg PG, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Iakovou I, Dangas G, Aquino MB, Sartori S, Chieffo A, Moliterno DJ, Colombo A, Mehran R. Incidence, Patterns, and Impact of Dual Antiplatelet Therapy Cessation Among Patients With and Without Chronic Kidney Disease Undergoing Percutaneous Coronary Intervention: Results From the PARIS Registry (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients). Circ Cardiovasc Interv. 2018;11:e006144.
49. Chen YT, Chen HT, Hsu CY, Chao PW, Kuo SC, Ou SM, Shih CJ. Dual Antiplatelet Therapy and Clinical Outcomes after Coronary Drug-Eluting Stent Implantation in Patients on Hemodialysis. Clin J Am Soc Nephrol. 2017;12:262-71.
50. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2015;10:1024-94.
51. Hemmelgarn BR, Southern D, Culleton BF, Mitchell LB, Knudtson ML, Ghali WA; Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) Investigators. Survival after coronary revascularization among patients with kidney disease. Circulation. 2004;110:1890-5.
52. Baber U, Farkouh ME, Arbel Y, Muntner P, Dangas G, Mack MJ, Hamza TH, Mehran R, Fuster V. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J. 2016; 37:3440-7.
53. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus- Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J Am Coll Cardiol. 2015;66:1209-20.
54. Zhang X, Hu L, Zheng W. Percutaneous Coronary Intervention versus Coronary Artery Bypass Graft in Acute Coronary Syndrome patients with Renal Dysfunction. Sci Rep. 2018;8:2283.
55. Roberts JK, Rao SV, Shaw LK, Gallup DS, Marroquin OC, Patel UD. Comparative Efficacy of Coronary Revascularization Procedures for Multivessel Coronary Artery Disease in Patients With Chronic Kidney Disease. Am J Cardiol. 2017;119:1344-51.
To download, please click below.