Introduction
Obstructive coronary arteries are the predominant cause of coronary artery disease (CAD), which is a major contributor of cardiac mortality and morbidity globally1. Stable angina, acute coronary syndromes, and silent myocardial ischaemia are the different types of CAD or ischaemic heart disease12. In the event of severe CAD symptoms, percutaneous coronary intervention (PCI) is considered as a suitable, minimally invasive treatment choice that treats the narrowing coronary arteries and enhances the supply of oxygenated blood to the coronary arteries. PCI with coronary angioplasty has undergone a paradigm shift from the times of plain old balloon angioplasty to the introduction of stents. Since bare metal stents were deemed less durable in the long term, the use of drug-eluting stents (DES) became widely accepted. Percutaneous transluminal coronary angioplasty (PTCA) with DES implantation has shown great success in the last decade for the treatment of different coronary lesions with or without calcification. However, the rates for procedural outcomes, such as in-stent restenosis (ISR) or lumen loss, and clinical outcomes, such as cardiovascular mortality and bleeding, reported in the current literature show that challenges remain in the current practice of interventional cardiology34. Therefore, presenting the advancements in stenting devices is warranted.
Noteworthily, a DES is well known for its abilities to control the development of neointimal hyperplasia and to contribute to neoendothelialisation following stent implantation45. Newer-generation stents with thinner struts, especially sirolimus-eluting stents (SES), have proven their efficacy in combatting the development of restenosis and late stent thrombosis (ST), which are linked to adverse prognostic implications and worsening CAD. The recent evidence on DES shows a marked reduction in the burden of mortality and improvement in the quality of life for patients undergoing PCI. With time, the new-generation thinner-strut stents have become the preference for ensuring successful PCI for various lesion types in the main coronaries as well as in the distal branches678. BioMime SES (Meril Life Sciences Pvt. Ltd., India) is one of the newer-generation SES platforms, with an ultrathin (65 μm) strut and a cobalt-chromium construction89. This next-generation SES has a novel hybrid design with closed (on the proximal and distal ends of the stent) and open cells (in the middle), which facilitate morphology-mediated expansion for adequate conformability, flexibility, and radial strength, with less chance of edge dissections9.
The series of publications of the meriT trials provide evidence on the efficacy and safety of the ultrathin BioMime SES in treating single or multiple de novo native coronary lesions, ISR, and bifurcation lesions. They report excellent procedural (100% in meriT-1 and meriT-3, 99.2% in meriT-2) and device success rates (99.41% in meriT-V), low rates of major adverse cardiovascular events (MACE; 0% in meriT-1, 6% in meriT-2, 2.35% in meriT-3, and 2.98% in meriT-V trials), and relatively low rates of in-stent late lumen loss (LLL) at the 8-month angiographic follow-up (median 0.15 mm [interquartile range IQR] 0.09-0.33] in meriT-1 and 0.12 mm [IQR 0.04-0.30] in meriT-2 trials)89101112. The 12-month clinical outcomes from the meriT-2 trial, along with 8-month angiographic follow-up data, showed high procedural and safety success rates, with notably low LLL10. In the meriT-V trial, the clinical performance of BioMime SES was investigated in comparison to the XIENCE V/Prime/Xpedition everolimus-eluting coronary stent systems (EECSS; Abbott)12. Abizaid et al reported similar rates of in-stent LLL at the 9-month angiographic follow-up (BioMime SES vs XIENCE EECSS: 0.15±0.27 mm vs 0.15±0.29 mm)12.
In continuation of the previous publication of 1-year outcomes of the meriT-2 trial, we report the clinical outcomes of patients who completed the 5-year follow-up after undergoing PCI with the implantation of BioMime SES.
Methods
STUDY POPULATION
The meriT-2 trial was a prospective, long-term, non-randomised, single-arm, multicentre study enrolling 250 patients at 11 investigational sites across India. The objective of this clinical study was to evaluate the safety, efficacy, and overall clinical performance of the BioMime SES system in the treatment of coronary lesions in CAD patients over a period of 5 years. The following inclusion criteria were applied for patient enrolment: patients aged ≥18 years, those eligible for PTCA with stenting, patients with symptomatic ischaemic heart disease, and those with obstructive CAD with Thrombolysis in Myocardial Infarction (TIMI) coronary flow grade ≥2. Patients in whom PTCA with stenting for chronic total occlusion (CTO) and bifurcation lesions was also permitted were included if they had de novo lesions and lesion lengths ≤35 mm that could be treated with a single stent (13 mm to 40 mm lengths) without the need for overlapping stents.
STUDY OUTCOME MEASURES
In continuation of the previous publication of the 12-month follow-up data of the meriT-2 trial, this publication is intended to present the long-term clinical follow-up data of the meriT-2 trial including MACE (comprising cardiac death, myocardial infarction [MI], emergent coronary artery bypass grafting [CABG], and clinically indicated target lesion revascularisation [CI-TLR], i.e., repeat PCI or CABG)10. ST was evaluated as per the Academic Research Consortium (ARC) definitions13.
The study was approved by the local ethics committees of all the participating centres, and the trial was performed under the principles stated in the Declaration of Helsinki. Written informed consent was provided by the enrolled patients before the index procedure. The meriT-2 trial was registered at the National Institute of Medical Statistics, Indian Council of Medical Research (Clinical Trials Registry – India, CTRI; www.ctri.nic.in/Clinicaltrials: CTRI/2016/11/007440) and at the US National Institute of Health (ClinicalTrials.gov: NCT02406326).
FOLLOW-UP
The follow-up dates were preplanned (5 years) from the date of the individual’s index procedure. In this article, the clinical outcomes data from 3- and 5-year follow-up visits are reported. The patients were assessed thoroughly for any symptoms of late ST or ISR as well as the need for CI-TLR through PCI or CABG surgery. The PCI procedures were performed in accordance with the standard American Heart Association (AHA) guidelines14.
STATISTICAL ANALYSIS
All data were recorded on case report forms. Categorical variables have been described as counts and percentages. Continuous variables have been described as mean±standard deviation (SD) in case of normal distribution and median with IQR (25-75%) in case of non-normal distribution of data. The safety and efficacy analyses were conducted on the basis of the intention-to-treat (ITT) principle. In this article, we are reporting the per-protocol analysis of the enrolled study population, whereas the ITT analysis was described in the previous publication of the same trial10. The ITT population included all patients meeting the eligibility criteria and undergoing the index PCI with the study device. The ITT analysis of 250 patients including the 12-month follow-up data has been reported earlier10. In this article, we report the per-protocol analysis of the 214 patients who completed the 5-year follow-up, including the Kaplan-Meier survival analysis of this study population, which was conducted to establish the MACE-free survival over 5 years. Comparisons between groups using 2-sided p-values were obtained using Fisher’s exact test or the χ2 test. The time-to-event analysis and overall survival analysis was performed using the Kaplan-Meier method; p-values were derived using the log-rank t-test. The lost-to-follow-up and withdrawn cases were censored when the survival analysis was performed. SPSS, version 20 (IBM) was used for all statistical analyses.
Results
The data of the 214 patients (85.6% of the 250 enrolled patients) who completed the 5-year follow-up visits were analysed. Figure 1 presents the CONSORT patient flow diagram of the trial. The baseline characteristics of these patients are presented in Table 1 . Briefly, the patient population had a mean age of 57.44±10.75 years and a mean body mass index of 25.11±3.52 kg/m2. A total of 82.71% of patients were males. Common cardiac risk factors such as diabetes and hypertension were present in 32.71% and 50.00% patients, respectively. The cardiac history evaluation revealed that 30.84% of patients had experienced a past (≥30 days) MI, while 24.76% had a recent MI (<30 days).
A total of 308 lesions were identified and treated with BioMime SES. Most of the treated lesions were in the left anterior descending artery (n=140, 45.46%), followed by the right coronary artery (n=101, 32.79%), and left circumflex artery (n=67, 21.75%). Type B2 lesions (n=138) were predominantly (44.81%) present, while 106 were type B1 lesions (34.42%), 52 were type C lesions (16.88%), and 12 lesions (3.89%) were of type A morphology (Table 2). The mean lesion length was 14.52±8.15 mm. The entire patient sample of the meriT-2 trial did not have any cases of CTO; however, 5.1% (n=18) of treated lesions were bifurcation lesions. In terms of clinical presentation, 10.74% patients presented with silent ischaemia, while 34.57% and 29.43% had stable and unstable angina, respectively. Preprocedural TIMI flow grade of 0 or 1 was present in 33.44% patients, while postprocedural TIMI flow grade 3 was achieved in the majority of the patients (98.7%).
Antiplatelet therapy was prescribed as per the investigator’s discretion and included aspirin (98.6%), clopidogrel (97.7%), prasugrel (0.47%), persantin (0.47%) and tirofiban (0.47%).
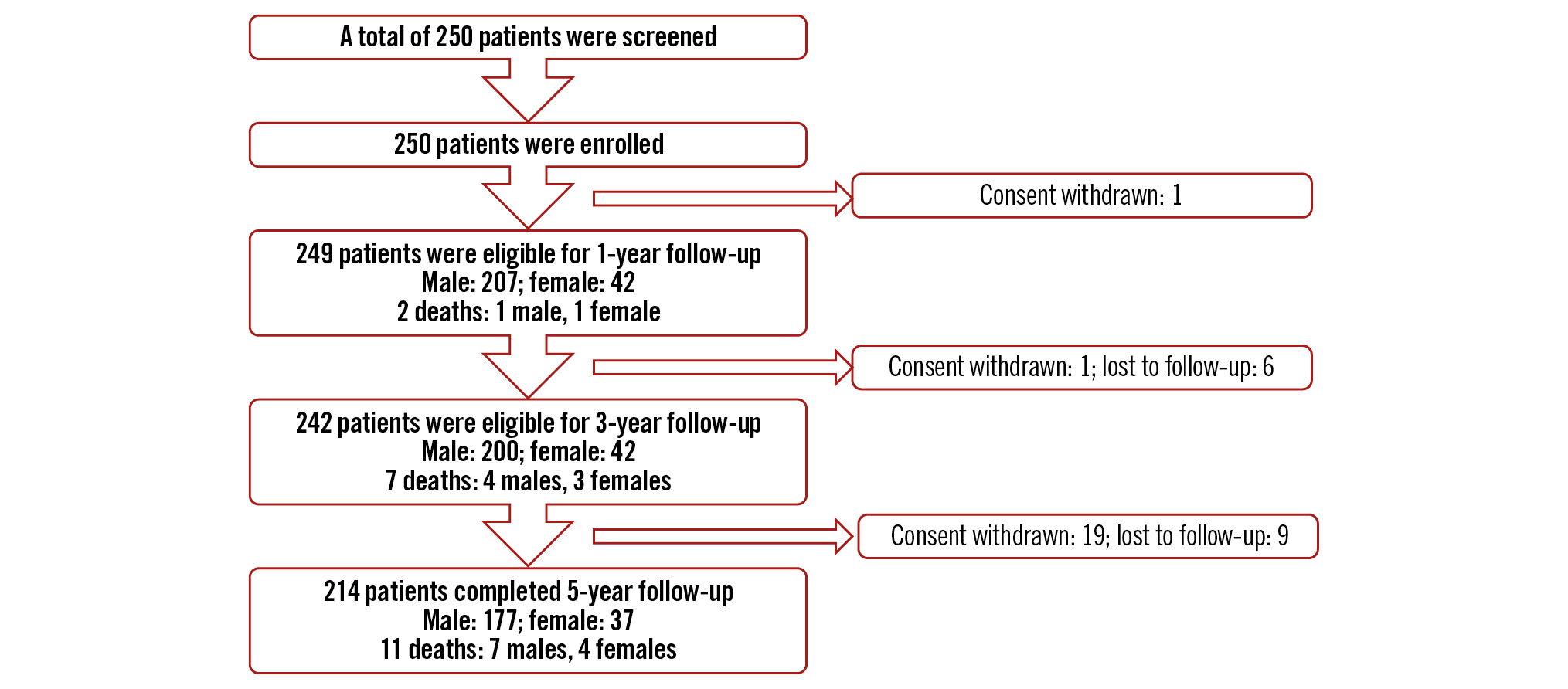
Figure 1. CONSORT flow diagram over the 5-year follow-up period. Index procedure performed on 249 patients.
Table 1. Baseline characteristics of the study population who completed 5-year follow-up.
| Variables | Enrolled study population (N=250) | Study population at 5-year follow-up (N=214) |
|---|---|---|
| Age, years | 56.8±10.6 | 57.44±10.75 |
| Sex | ||
| Female | 42 (16.8) | 37 (17.29) |
| Male | 208 (83.2) | 177 (82.71) |
| Diabetes mellitus | 91 (36.4) | 70 (32.71) |
| Hypertension | 123 (49.2) | 107 (50.00) |
| Dyslipidaemia | 26 (10.4) | – |
| Prior MI (≥30 days) | 80 (32.0) | 66 (30.84) |
| Prior PCI | 15 (6.0) | 11 (5.14) |
| Prior CABG | 4 (1.6) | 3 (1.40) |
| Prior stroke/TIA/CVA | 2 (0.8) | 2 (0.93) |
| History of CHF | 5 (2.0) | 3 (1.40) |
| Clinical presentation | ||
| Asymptomatic/silent ischaemia | 28 (11.2) | 23 (10.74) |
| Stable angina | 94 (37.6) | 74 (34.57) |
| Unstable angina | 68 (27.2) | 63 (29.43) |
| Recent MI (<30 days) | 59 (23.6) | 53 (24.76) |
| Values are mean±standard deviation or n (%). CABG: coronary artery bypass grafting; CHF: congestive heart failure; CVA: cerebrovascular accident; MI: myocardial infarction; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack | ||
Table 2. Lesion characteristics of the patients with 5-year follow-up.
| Variable | Total no. of lesions (n=308) |
|---|---|
| Target coronary vessel | |
| LD | 140 (45.46) |
| LCx | 67 (21.75) |
| RCA | 101 (32.79) |
| Lesion class# | |
| A | 12 (3.89) |
| B1 | 106 (34.42) |
| B2 | 138 (44.81) |
| C | 52 (16.88) |
| Values are presented as n (%). #According to the modified American College of Cardiology/American Heart Association classification criteria. LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery | |
CLINICAL OUTCOMES UP TO 3 YEARS
A total of 242 patients completed the 3-year follow-up. The MACE rate was 7.9% (cardiac death in 0.8%, MI in 1.7%, and CI-TLR in 5.4% of the patients). Definite/probable ST had occurred in 1 patient (0.4%) at 6-month follow-up, and no new cases of ST were reported during the 3-year follow-up (Table 3, Figure 2). The cumulative frequency of all-cause death was 3.7%, including 2 cardiac and 7 non-cardiac deaths, at 3 years. Repeat revascularisation was required in 13 patients (5.4%), including 1 patient who underwent CABG (Table 3).
Table 3. Cumulative data of the clinical outcomes of the study population at 3- and 5-year follow-up.
| 3-year follow-up (N=242) | 5-year follow-up (N=214) | p-value | |
|---|---|---|---|
| MACE | 19 (7.9) | 19 (8.9) | 0.8209 |
| Cardiac death | 2 (0.8) | 2 (0.9) | 1.0000 |
| MI | 4 (1.7) | 4 (1.9) | 1.0000 |
| Clinically indicated TLR | 13 (5.4) | 13 (6.1) | 0.9039 |
| PCI | 12 (5.0) | 12 (5.6) | 0.9207 |
| CABG | 1 (0.4) | 1 (0.5) | 1.0000 |
| Non-cardiac death | 7 (2.9) | 9 (4.2) | 0.6132 |
| Stent thrombosis* | 1 (0.4) | 1 (0.5) | 1.0000 |
| Definite/probable | 1 (0.4) | 1 (0.5) | 1.0000 |
| Possible | 0 | 0 | – |
| Values are n (%). *According to the ARC definitions. ARC: Academic Research Consortium; CABG: coronary artery bypass grafting; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation | |||
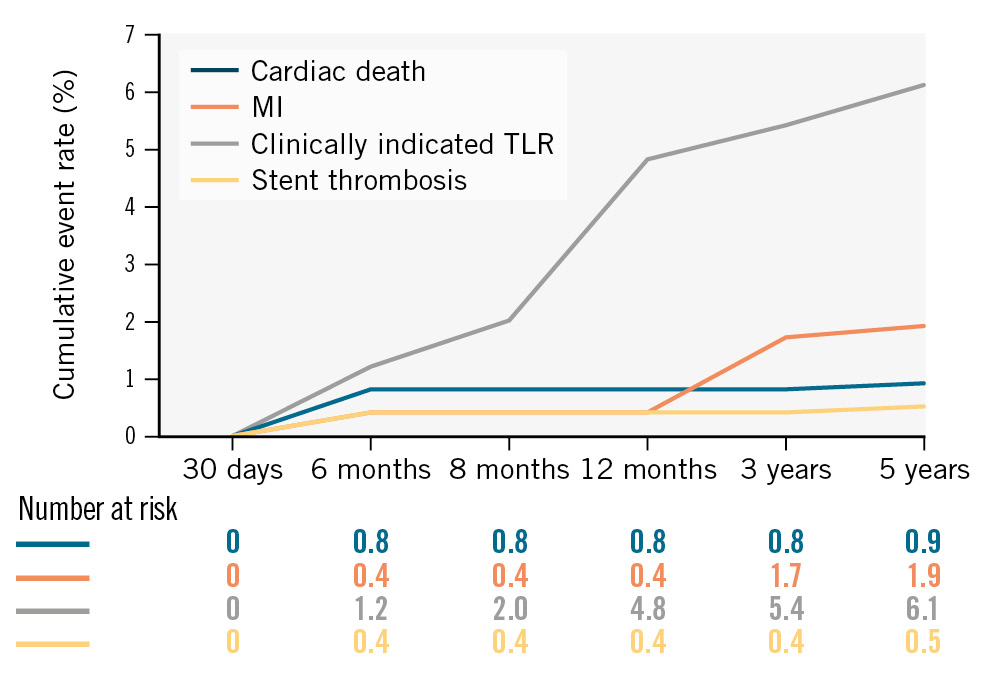
Figure 2. Cumulative clinical outcomes of the meriT-2 trial up to 5-year follow-up. MI: myocardial infarction; TLR: target lesion revascularisation
CLINICAL OUTCOMES UP TO 5 YEARS
A total of 214 patients completed the 5-year follow-up. MACE was reported in 8.9% of patients (cardiac death in 0.9%, MI in 1.9%, and CI-TLR in 6.1% of the patients) (Figure 2). The rates of possible ST and definite/probable ST were considerably low, and no new cases were observed during the 5-year follow-up period (Table 3). Between the 3-year and 5-year follow-ups, no other all-cause mortality events had occurred in female patients, while 2 more males died. Hence, a total of 7 men and 4 women died during 5-year follow-up. The actuarial MACE-free survival obtained with the Kaplan-Meier analysis was 95.6% over the 5-year study period for the ITT population (Central illustration).
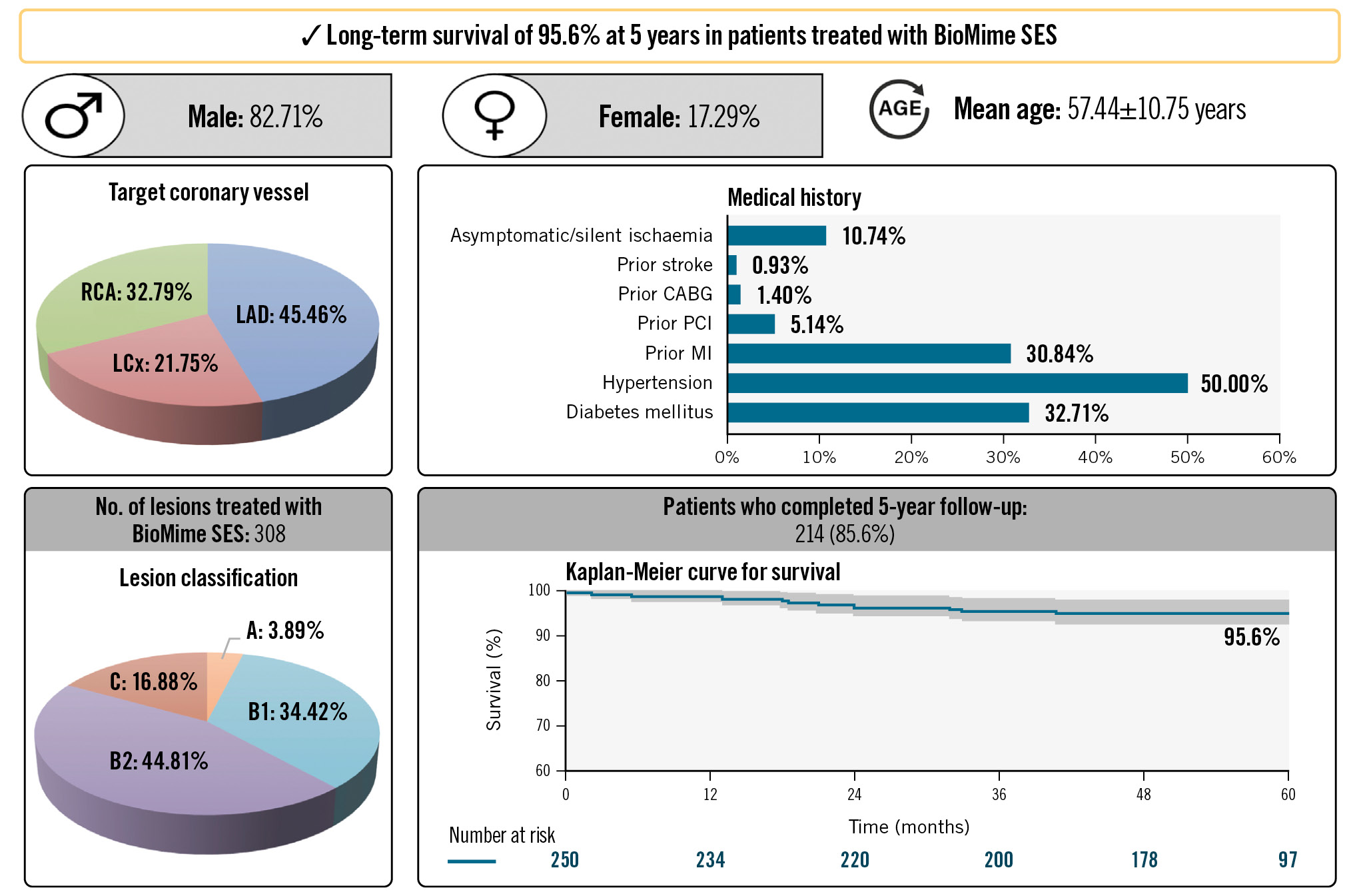
Central illustration. Long-term follow-up data of the meriT-2 trial reveal the safety and efficacy of the BioMime SES. CABG: coronary artery bypass grafting; LAD: left anterior descending artery; LCx: left circumflex artery; MI: myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery; SES: sirolimus-eluting stent
Discussion
In this report, we discuss the long-term data of 214 patients who completed the 5-year follow-up after undergoing PCI with the implantation of BioMime SES. The first key finding of the meriT-2 trial is that the cumulative frequency of late ST (definite or probable) at the 5-year follow-up was quite low (0.5%). Secondly, no cardiac deaths were reported in the 5-year study period (cumulative MACE rate at 5 years: 8.9%), which is noteworthy, given the study sample’s significant comorbidity burden and history of acute coronary events. With the 5-year clinical outcome data available for the study, we affirm that the meriT-2 trial reports satisfactory levels of long-term efficacy and safety of the BioMime SES for the treatment of de novo lesions in patients with obstructive CAD. In this study, the actuarial MACE-free survival at 5 years was 95.6% based on Kaplan-Meier analysis.
The first-in-human evaluation of the BioMime SES was conducted in the meriT-1 study, which reported 0% MACE (composite of MI, cardiac death, TLR) and ST rates. The overall results from the meriT-1 trial demonstrated excellent performance and safety outcomes at the 12-month follow-up with high procedural success and a low median in-stent LLL (0.15 mm [IQR 0.09-0.33]) at the 8-month angiographic follow-up9. The results of the meriT-2 trial reiterate the competency of the BioMime SES based on the previously reported 1-year data reported by Seth et al (2016)10. It was shown that the cumulative MACE rate was 6%, including 0.4% MI, 4.8% CI-TLR, and 0.8% cardiac death. The reported meriT-2 trial data highlight the feasibility of implanting the BioMime SES, as 99.2% angiographic and procedural success was achieved10. Furthermore, Seth et al reported that at the 8-month angiographic follow-up, the median in-stent LLL was 0.12 mm (IQR 0.04-0.30), suggesting the considerable ability of the study device in inhibiting neointimal hyperplasia and preventing restenosis10. The low rate of ST and high MACE-free survival rate achieved in the study population at 5 years corroborate data regarding the efficacy of the BioMime SES. In continuation of the meriT series, the meriT-V trial has demonstrated remarkable efficacy in not only achieving low in-segment LLL (0.12±0.26 mm) and in-stent LLL (0.15±0.27 mm) at 9 months post-PCI, as reported by Abizaid et al (2018), but also in reducing long-term adverse events including MACE, ischaemia-driven target lesion revascularisation (ID-TLR), ischaemia-driven target vessel revascularisation (ID-TVR), cardiac death, and late ST12. The meriT-V trial demonstrated good efficacy of the BioMime SES in terms of lowering the repeat revascularisation rates during the 2-year follow-up15. The quantitative coronary angiographic analysis of the meriT-V trial at the 9-month angiographic follow-up showed the non-inferiority of the BioMime SES in comparison to XIENCE V/Prime/Xpedition EECSS in terms of the primary endpoint data, as similar in-stent LLL rates were observed with both the devices (BioMime SES vs XIENCE EECSS: 0.15±0.27 mm vs 0.15±0.29 mm) at the 9-month angiographic follow-up12. In addition, neither of the groups had any significant difference in the percentage of diameter stenosis (BioMime SES vs XIENCE EECSS: 16.82±11.90% vs 16.57±11.71%) at the 9-month follow-up12.
Furthermore, the clinical outcomes of the meriT-2 trial are comparable to those reported from the DESSOLVE III trial (Table 4)16. The role of a DES in the treatment of ISR or de novo coronary lesions involves multiple mechanisms that contribute to re-endothelisation and suppression of vascular remodelling. Inhibition of stenotic vascular remodelling is based on the action of the antiproliferative drug (sirolimus), which inhibits the growth and proliferative activity of vascular smooth muscle cells that migrate and proliferate into the intimal or medial layers of the arterial wall and cause stiffening. The rapid re-endothelisation promoted by the action of sirolimus over a span of 30 days by a sustained or extended release causes a stark decrease in the risk of ISR1017.
The study device, BioMime SES, is known for its excellent conformability to the arterial lumen. The fusion of open and closed cells into a hybrid structure enables a better vessel conformity and offers a higher efficiency in-stent deployment owing to the presence of the ultrathin 65 μm struts. Other stents with an ultrathin construction have also shown similar results (Table 5). The device is known for its abilities to reduce balloon-related vascular edge injuries and offer complete resorption of the biopolymeric coating within 30 days18. From the outcomes of the meriT-2 trial, the advantages of the ultrathin-strut design of the BioMime SES are demonstrated as early and late safety events, which were lower than those reported in contemporary studies (Table 5). However, consolidated meta-analyses of the data in future investigations are warranted to fully ascertain the clinical equivalence of the study device with the other commercially available devices.
Nevertheless, we believe that these improved outcomes may be attributed to the presence of a highly biocompatible and biodegradable polymer composite comprising two biodegradable polymers – poly-L-lactide (PLLA) and poly-DL-lactide-co-glycolide (PLGA). In comparison with contemporary studies on ultrathin-strut SES designs, the meriT-2 trial presents considerable evidence on the BioMime SES in terms of low cardiac deaths and CI-TLR, which ultimately reduce the MACE rates. The MACE rates reported in this study (8.9%) are comparable to the rates of device-oriented composite endpoints or MACE shown for the PROMUS everolimus-eluting stent (Boston Scientific), MiStent SES (Micell Technologies) and XIENCE EECSS (Table 5)1619.
The long-term outcomes data of the meriT-2 trial present satisfactory PCI outcomes up to 5 years, which validate the performance of the BioMime SES in patients with obstructive CAD. The repeat revascularisation rate with PCI (n=12, 5.6%) and CABG (n=1, 0.5%) during the 5-year follow-up was relatively low. Furthermore, we noted that the revascularisation rate was comparable to that observed in the LEADERS FREE III study (Table 5)20. An independent patient-data meta-analysis compared 5 randomised controlled trials, BIOFLOW-II, BIOFLOW-IV, BIOFLOW-V, BIOSCIENCE and BIOSTEMI, to evaluate the safety and efficacy of ultrathin-strut biodegradable-polymer sirolimus-eluting stents (BP-SES) and thin-strut durable-polymer everolimus-eluting stents (DP-EES). Among both the groups, there were no significant differences between BP-SES and DP-EES with regard to cardiac death (3.4% vs 4.1%), all-cause mortality (6.4% vs 6.4%) or revascularisation (11.8% vs 12.5%). These 5 randomised controlled trials demonstrated a similar risk of TLF among patients undergoing PCI with BP-SES compared to those undergoing PCI with DP-EES21. Thus, the data from the meriT-2 trial provide evidence of satisfactory clinical outcomes at long-term follow-up. It is noteworthy that there were no restrictions on the inclusion of females in the study, which helped assimilate the outcomes for female patients in a middle-income nation as well. The infrastructural developments and improvements in socioeconomic factors helped assess the PCI outcomes across both sexes and across multiple age groups.
Table 4. Recently published clinical studies from contemporary devices conducted with thin-strut DES.
| Study names | Study design | Study device |
|---|---|---|
| DESSOLVE III trial16 | Prospective, multicentre, single-blinded, all-comers, randomised controlled trial | MiStent SES (64 μm)a and XIENCE EECSS (strut thickness of 81 μm)b |
| Genoss DES Prospective Multicenter Registry22 | Prospective, single-arm observational, multicentre registry | GENOSS DES SES (70 µm)c |
| Historical cohort study19 | Retrospective, historical cohort study | PROMUSd/Resolutee/ XIENCEb (PRX) and BioMimef |
| BIOFLOW-VII23 | Prospective, multicentre, single-arm US post-marketing approval study | Orsiro SES (60 µm)g |
| Thailand Orsiro Registry24 | Prospective, non-randomised, multicentre, observational study | Orsiro SES (60 µm)g |
| Leaders Free III study20 | Prospective, international, multicentre, single-arm study | BioFreedom biolimus-A9-eluting stent (84-88 μm)h |
| FlexyRap DES study25 | Retrospective, single-arm, multicentre, post-marketing study | FlexyRap cobalt-chromium rapamycin-eluting stent (60 µm)i |
| aBy Micell Technologies; bby Abbott; cby Genoss; dby Boston Scientific; eby Medtronic; fby Meril Life Sciences; gby Biotronik; hby Biosensors International; iby SLTL Medical. DES: drug-eluting stent; EECSS: everolimus-eluting coronary stent system; SES: sirolimus-eluting stent | ||
Table 5. Recently published clinical outcomes from contemporary studies conducted with thin-strut DES.
| Study names | No. of patients and follow-up | Cardiac death | Any TLR/CD-TLR/CI-TLR | Any TVR/CD-TVR/non-CD-TVR | MI | TVMI | Late ST | ST |
|---|---|---|---|---|---|---|---|---|
| DESSOLVE III trial [16} | N=13983 years | MiStenta vs XIENCE EECSSb: 3.9% vs 3.8% | MiStent vs XIENCE EECSS (CD-TLR): 5.2% vs 6.5% | MiStent vs XIENCE EECSS (any TVR): 8.7% vs 10.2% | MiStent vs XIENCE EECSS (any MI): 3.5% vs 3.2% | MiStent vs XIENCE EECSS: 3.2% vs 2.5% | MiStent vs XIENCE EECSS: 0.4% vs 0.6% | MiStent vs XIENCE EECSS (definite ST): 0.6% vs 1.2% |
| Genoss DES Prospective Multicenter Registry22 | N=6221 year | 0.2% | 0.5% | 0.8% | Any MI: 0.6% | 0.2% | 0.5% | Definite ST: 0.5% Probable ST: 0.6% |
| Historical cohort study19 | N=17091 year | PRX vs BioMime SESe group vs overlap (1.5% vs 2.4% vs 1.1%) | PRX vs BioMime SES group vs overlap (1.6% vs 0.8% vs 1.9%) | PRX vs BioMime SES group vs overlap (2.8% vs 1.6% vs 3.2%) | – | – | – | – |
| BIOFLOW-VII23 | N=5561 year | 0% | 0.9 | 2.3% | Any MI: 1.7% | 1.3% | – | Definite ST: 0.4%Probable ST: 0% |
| Thailand Orsiro Registry24 | N=1501 year | 5.3% | – | 0.7% | Any MI: 1.3% | 0% | – | Definite ST: 0.7%Probable ST: 0.7% |
| Leaders Free III study20 | N=4011 year | 3.7% | 4.2% | 5.0% | 4.4% | – | – | Definite/probable ST: 1.0% |
| FlexyRap DES study25 | N=5005 years | 0% | 0% | 0% | – | 0.2% | Late ST: 0%Very late ST:0% | – |
| aBy Micell Technologies; bby Abbott; cby Boston Scientific; dby Medtronic; eby Meril Life Sciences. CD-TLR: clinically driven TLR; CD-TVR: clinically driven TVR; CI-TLR: clinically indicated TLR; DES: drug-eluting stent; EECSS: everolimus-eluting coronary stent system; MI: myocardial infarction; PRX: PROMUSc/Resoluted/XIENCEb; SES: sirolimus-eluting stent; ST: stent thrombosis; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction; TVR: target vessel revascularisation | ||||||||
Limitations
We noted that the stringent exclusion criteria limited the inclusion of real-world patients, and the lack of a heterogeneous ethnic population in this study may limit its generalisability for the larger proportion of PCI patients in real-world practice. The absence of a comparative arm in the meriT-2 trial needs to be acknowledged. Moreover, long-term coronary angiographic data would be necessary to ascertain the durability of the biodegradable-polymer-based cobalt-chromium stent, BioMime SES. In the current era of PCI, the need for late angiographic follow-up has been reduced owing to the consistent decrease in ST events. In selected cases, angiographic follow-up may be conducted when the clinical findings indicate the need for an invasive examination.
Conclusions
Long-term follow-up data of the meriT-2 trial reveal the safety and efficacy of the BioMime SES in the treatment of obstructive CAD with the absence of late ST and low frequency of MACE over the 5-year study period. Long-term survivorship of 95.6% at 5 years was demonstrated in patients treated with the BioMime SES, despite these patients having multiple comorbidities.
Impact on daily practice
These real-world long-term outcomes at 5 years with the BioMime sirolimus-eluting stent will impact the daily practices of interventional cardiologists in optimising their treatment of patients with obstructive coronary artery disease. For researchers, the antiproliferative activity of sirolimus on the stent which, due to rapid re-endothelisation, reduces in-stent restenosis, in turn, improving long-term outcomes, would be of interest.
Data availability statement
The study data are available from the corresponding author upon reasonable request.
Guest Editor
This paper was guest edited by Davide Capodanno, MD, PhD; A.O.U. Policlinico “G. Rodolico-San Marco”, University of Catania, Catania, Italy
Acknowledgements
We would like to thank the biostatisticians, Mr Sayyed Israr Ahamad, Ms Rutuja D. Sadekar, and Mr Vishal Jagadale, at Meril Life Sciences Pvt. Ltd., India, for their tireless efforts and support throughout data analysis and in obtaining survival analysis data. We also extend our gratitude to Ms Vibhuti S. Bhatt, Ms Mahasweta G. Pal, and Dr Latheef Kasala for their assistance in finalising the manuscript.
Funding
The meriT-2 trial was funded by Meril Life Sciences Pvt. Ltd., Gujarat, India.
Conflict of interest statement
A. Thakkar and U. Chandra are full-time employees of Meril Life Sciences Pvt. Ltd. U. Kaul is the Editor-in-Chief of AsiaIntervention. The other authors have no conflicts of interest to declare. The Guest Editor has no relevant conflicts of interest to declare.

