Introduction
Drug-coated balloon (DCB) angioplasty has emerged as an effective treatment option for de novo coronary artery lesions. The clinical outcomes following treatment with DCB angioplasty are not inferior to those with drug-eluting stents (DES)1. Previous studies have reported that the angiographic classification of stent restenosis is prognostically important234, although the chronic-phase angiographic patterns after DCB angioplasty for de novo lesions have not yet been described. Furthermore, the distribution of these patterns is unclear. Therefore, the aim of the present study was to evaluate chronic-phase angiographic classification after DCB angioplasty.
Materials and methods
This study was a retrospective, observational study conducted at a single centre. Between June 2016 and August 2022, a total of 670 patients with 708 de novo coronary lesions underwent DCB angioplasty. A successful DCB angioplasty was defined as a non-flow-limiting dissection, with a residual stenosis ≤30% and absence of a bailout stent. A drug-coated balloon (SeQuent Please [B. Braun]) was used for the procedure. Patients with suboptimal results (residual stenosis >30%), acute target lesion thrombosis, and those without follow-up coronary angiography (CAG) after DCB angioplasty were excluded from the study. All patients were consulted about having angiographic follow-up, and those who consented underwent a planned angiogram 6 to 12 months later. Alternatively, CAG was performed on patients if they complained of symptoms suggestive of angina pectoris within 12 months after DCB angioplasty. Consequently, only 318 patients with 337 lesions were included in this study (Figure 1). In total, 312 lesions (92.6%) were evaluated by a planned angiography. Meanwhile, a symptom-driven angiography was performed for 25 lesions (7.4%). There were two emergent angiograms. The baseline clinical, lesion, and procedural characteristics of the patients are presented in Table 1 and Table 2. Table 2 shows the final balloon used. If sufficient lumen was not obtained after dilatation with the initially used balloon, an additional dilatation was performed using a larger and/or scoring balloon. Several balloons were used in 102 lesions (30.2%). The most common final balloon used was a scoring balloon or cutting balloon (84.6%). An intracoronary imaging device was used in 311 lesions (92.3%). Bailout stent criteria recommended by the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT) expert consensus document were enforced. Medial dissection, intramural haematoma, or extra-medial injury was fixed with bailout stenting to avoid acute coronary closure in this institution. The Ethics Committee of Hokkaido Cardiovascular Hospital approved this study, which was carried out according to the 1975 Declaration of Helsinki guidelines. Before participation, informed consent was obtained from all eligible patients.
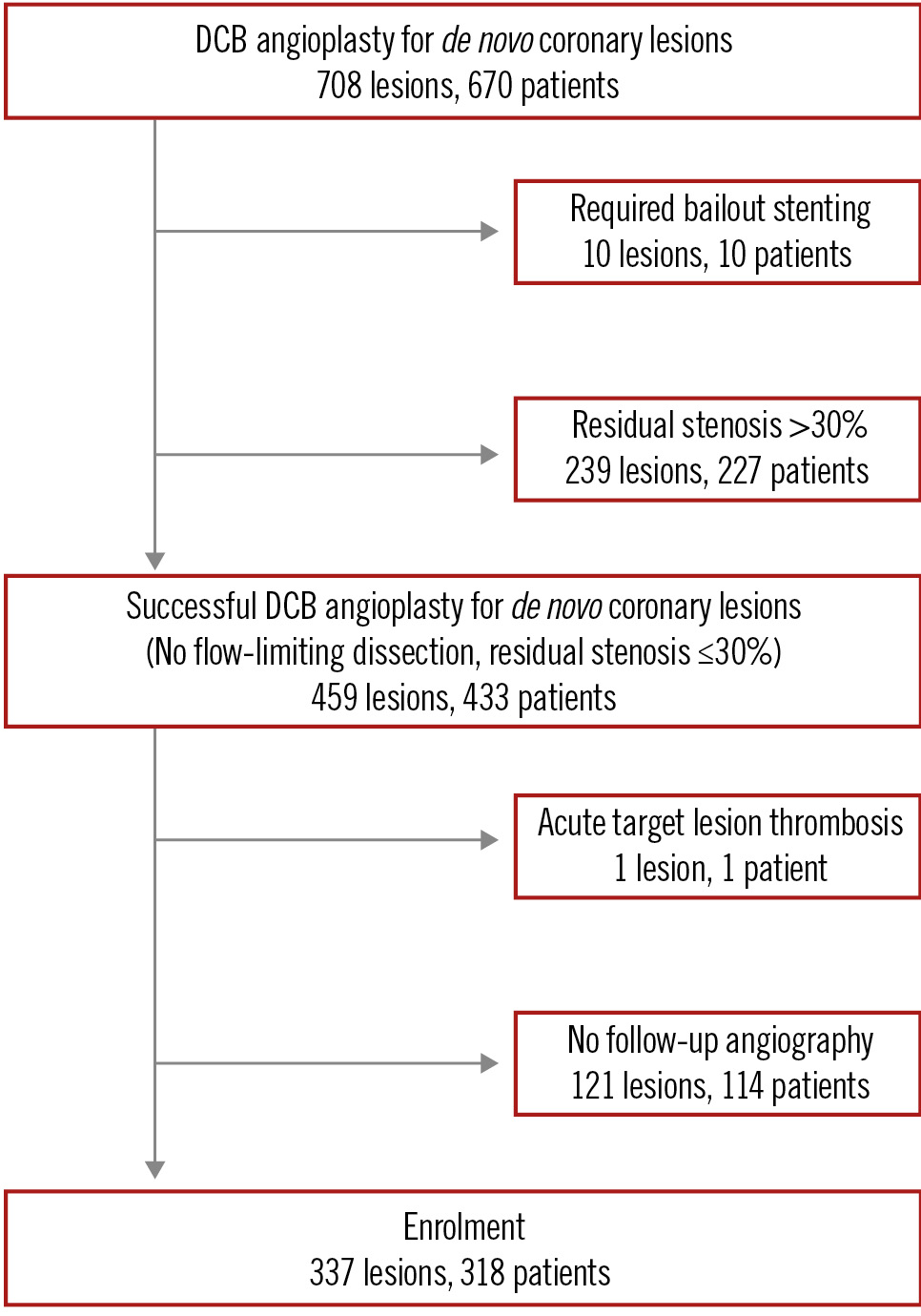
Figure 1. Study schema. DCB: drug-coated balloon
Table 1. Baseline clinical characteristics.
| Overall (n=318) | |
|---|---|
| Age, years | 68.2±11.1 |
| Male | 246 (77.4) |
| Hypertension | 245 (77.0) |
| Diabetes mellitus | 132 (41.5) |
| Dyslipidaemia | 246 (77.4) |
| Smoking history | 190 (59.7) |
| HD | 28 (8.8) |
| CKD | 55 (17.3) |
| Index presentation | |
| Stable angina | 221 (69.5) |
| ACS | 97 (30.5) |
| Prior PCI | 98 (30.8) |
| Prior CABG | 10 (3.1) |
| Prior MI | 60 (18.9) |
| Values are mean±standard deviation or n (%). ACS: acute coronary syndrome; CABG: cardiac artery bypass graft; CKD: chronic kidney disease; HD: haemodialysis; MI: myocardial infarction; PCI: percutaneous coronary intervention | |
Table 2. Lesion and procedural characteristics.
| Overall (n=337) | |
|---|---|
| Target vessels | |
| LAD | 149 (44.2) |
| LCx | 82 (24.3) |
| RCA | 104 (30.9) |
| LMCA | 2 (0.6) |
| AHA type B2/C | 193 (57.3) |
| Angiographic calcification | 69 (20.5) |
| Ostial lesion | 38 (11.3) |
| CTO | 12 (3.6) |
| Bifurcation | 58 (17.2) |
| Before procedure | |
| Lesion length, mm | 14.3±7.5 |
| RVD, mm | 2.34±0.55 |
| MLD, mm | 0.69±0.48 |
| %DS, % | 70.8±18.8 |
| After procedure | |
| RVD, mm | 2.41±0.51 |
| MLD, mm | 1.90±0.42 |
| %DS, % | 20.8±7.4 |
| Dissection (No/A/B/C) | 279 (82.8) / 14 (4.2) /39 (11.6) / 5 (1.5) |
| Follow-up | |
| Follow-up duration, days | 215±119 |
| RVD, mm | 2.47±0.51 |
| MLD, mm | 1.82±0.51 |
| %DS, % | 26.1±15.4 |
| LLL, mm | 0.09±0.41 |
| Dissection (No/A/B/C) | 336 (99.7) / 0 (0) /1 (0.3) / 0 (0) |
| DCB size | |
| DCB diameter, mm | 2.70±0.45 |
| DCB length, mm | 19.7±7.4 |
| Maximum inflation pressure, atm | 7.6±2.4 |
| Duration of inflation, s | 54.7±11.5 |
| Predilatation performed | 337 (100) |
| Conventional balloon | 44 (13.1) |
| Scoring/cutting balloon | 285 (84.6) |
| High pressure balloon | 8 (2.4) |
| Balloon diameter, mm | 2.61±0.47 |
| Balloon length, mm | 12.8±2.1 |
| Maximum inflation pressure, atm | 11.7±4.5 |
| Rotablator | 5 (1.5) |
| DCA | 1 (0.3) |
| Excimer laser | 14 (4.2) |
| Intracoronary imaging-guided PCI | 311 (92.3) |
| Values are mean±standard deviation or n (%). %DS: percentage diameter stenosis; AHA: American Heart Association; CTO: chronic total occlusion; DCA: directional coronary atherectomy; DCB: drug-coated balloon; LAD: left anterior descending artery; LCx: left circumflex artery; LLL: late lumen loss; LMCA: left main coronary artery; MLD: minimal lumen diameter; PCI: percutaneous coronary intervention; RCA: right coronary artery; RVD: reference vessel diameter | |
ANGIOGRAPHIC CLASSIFICATION AT FOLLOW-UP AFTER DCB ANGIOPLASTY
Angiographic patterns at follow-up were classified as follows (Figure 2):
Non-restenosis patterns
- Class I: non-restenosis. Stenosis is ≤50%.
- Class II: lumen enlargement. The minimal lumen diameter (MLD) on chronic-phase angiography was greater than the MLD immediately after DCB angioplasty.
Restenosis patterns
- Class III: focal restenosis. Stenosis is >50%. The lesions were approximately 10 mm in length.
- Class IV: diffuse restenosis. Stenosis is >50%. The lesions were >10 mm long.
- Class V: occlusive restenosis.
Additional findings
- Type A: aneurysm. This was defined as an abnormal coronary dilatation exceeding the diameter of the normal segment by at least 50%.
- Type B: balloon-edge restenosis. Stenosis was defined as stenosis within 5 mm of the border of the DCB-treated lesion, both distally and proximally.
- Type C: plaque cavity. This was defined as contrast staining outside the surface of the vascular lumen <50% of the diameter of the normal segment.
- Type D: residual dissection.
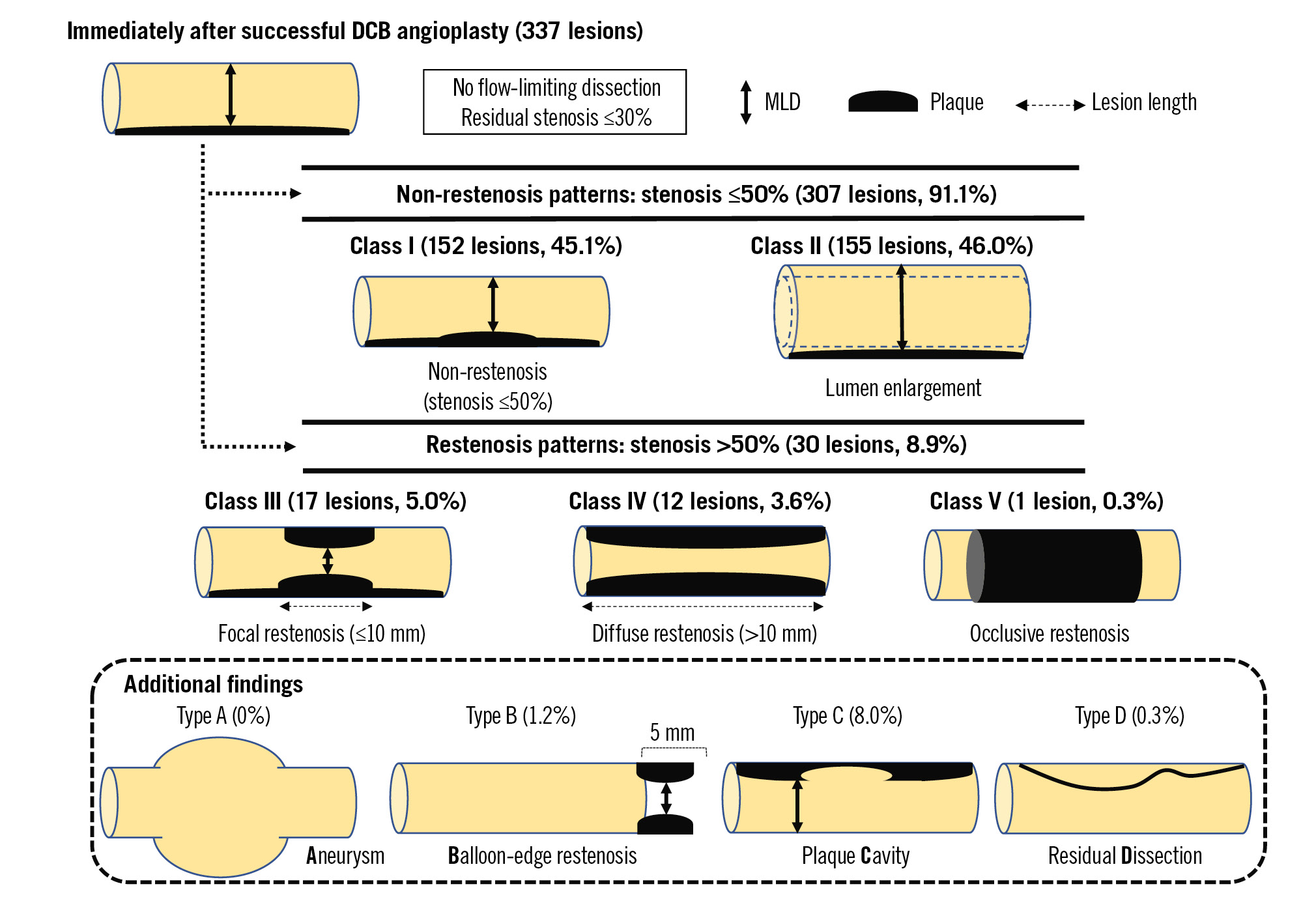
Figure 2. Schematic images of angiographic classification at follow-up after DCB angioplasty for de novo coronary lesions. DCB: drug-coated balloon; MLD: minimal lumen diameter
ANGIOGRAPHIC ANALYSIS
We performed preprocedural, postprocedural, and follow-up serial coronary angiography. Angiographic follow-up was not mandatory; it was performed for recurrent symptoms or as part of the routine angiographic follow-up if patients consented. We performed a quantitative coronary analysis (QCA) of the coronary angiographic data using the CAAS II Research System (Pie Medical Imaging) for each angiogram. Lesion length, reference vessel diameter (RVD), MLD, and percentage diameter stenosis (%DS) were measured. We calculated late lumen loss (LLL) as the postprocedural MLD minus the follow-up MLD. Angiographic calcification was identified as a readily apparent radiopacity within the vascular wall at the stenosis site. Angiographic restenosis was defined as a diameter stenosis of >50% of the treated lesion at follow-up. QCA was performed by experts at Hokkaido Cardiovascular Hospital who were blinded to the patient data.
Results
The mean follow-up period was 215±125 days. Of the 337 lesions analysed, 91.1% (n=307) were in the non-restenosis group, and 8.9% (n=30) were in the restenosis group. The non-restenosis group was classified as having non-restenosis (class I, 45.1%; n=152) or lumen enlargement (class II, 46.0%; n=155). The restenosis group was classified into focal restenosis (class III, 5.0%; n=17), diffuse restenosis (class IV, 3.6%; n=12), and occlusive restenosis (class V, 0.3%; n=1) (Table 3). In the additional findings, there were no aneurysms, and plaque cavities were often observed (8.0%). The rate of balloon-edge restenosis was 1.2%. Residual dissection in the chronic phase was only observed in one case (0.3%). The baseline clinical, lesion, and procedural characteristics are presented in Table 4 and Table 5.
Table 3. Angiographic classification.
| Angiographic classification (n=337) | ||||
|---|---|---|---|---|
| Non-restenosis: 307 (91.1) | Restenosis: 30 (8.9) | |||
| Class I | Class II | Class III | Class IV | Class V |
| 152 (45.1) | 155 (46.0) | 17 (5.0) | 12 (3.6) | 1 (0.3) |
| Additional findings (type A/B/C/D) in each class | ||||
| Class I | Class II | Restenosis (Class III, IV, V) | ||
| 0/1/7/1 | 0/2/15/0 | 0/1/5/0 | ||
| Additional findings (type A/B/C/D) in all cases | ||||
| Type A:0 (0) | Type B:4 (1.2) | Type C:27 (8.0) | Type D:1 (0.3) | |
| Values are n (%). | ||||
Table 4. Baseline clinical characteristics.
| Non-restenosis | Restenosis | p-value | |||
|---|---|---|---|---|---|
| Class I(n=152) | Class II(n=155) | Class III, IV, V (n=30) | Class I vs Class II vs restenosis | Non-restenosis vs restenosis | |
| Age, years | 68.7±10.7 | 67.3±11.5 | 69.2±11.6 | 0.460 | 0.556 |
| Male | 113 (74.3) | 122 (78.7) | 27 (90.0) | 0.157 | 0.091 |
| Hypertension | 118 (77.6) | 121 (78.0) | 21 (70.0) | 0.618 | 0.328 |
| Diabetes mellitus | 63 (41.4) | 64 (41.3) | 15 (50.0) | 0.658 | 0.361 |
| Dyslipidaemia | 112 (73.6) | 130 (85.5) | 20 (66.7) | 0.031 | 0.126 |
| Smoking history | 88 (57.9) | 99 (63.9) | 18 (60.0) | 0.560 | 0.922 |
| HD | 16 (10.5) | 10 (6.5) | 2 (3.3) | 0.409 | 0.733 |
| CKD | 27 (17.8) | 24 (15.5) | 7 (23.3) | 0.564 | 0.352 |
| Index presentation | 0.231 | 0.122 | |||
| Stable angina | 110 (72.4) | 107 (69.0) | 17 (56.7) | ||
| ACS | 42 (27.6) | 48 (31.0) | 13 (43.3) | ||
| Prior PCI | 50 (32.9) | 45 (29.0) | 9 (30.0) | 0.760 | 0.915 |
| Prior CABG | 3 (2.0) | 7 (4.5) | 1 (3.3) | 0.456 | 0.982 |
| Prior MI | 28 (18.4) | 31 (20.0) | 7 (23.3) | 0.812 | 0.588 |
| DAPT | 149 (98.0) | 152 (98.1) | 30 (100) | 0.742 | 0.440 |
| Values are mean±standard deviation or n (%). ACS: acute coronary syndrome; CABG: cardiac artery bypass graft; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; HD: haemodialysis; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||||
Table 5. Lesion and procedural characteristics.
| Non-restenosis | Restenosis | p-value | |||
|---|---|---|---|---|---|
| Class I(n=152) | Class II(n=155) | Class III, IV, V (n=30) | Class I vs Class II vs restenosis | Non-restenosis vs restenosis | |
| Target vessels | 0.322 | 0.734 | |||
| LAD | 58 (38.2) | 77 (49.7) | 14 (46.7) | ||
| LCx | 36 (23.7) | 37 (23.9) | 9 (30.0) | ||
| RCA | 57 (37.5) | 40 (25.8) | 7 (23.3) | ||
| LMCA | 1 (0.6) | 1 (0.6) | 0 (0) | ||
| AHA type B2/C | 86 (56.6) | 87 (56.1) | 20 (66.7) | 0.550 | 0.276 |
| Angiographic calcification | 29 (19.1) | 33 (21.2) | 7 (23.3) | 0.820 | 0.684 |
| Ostial lesion | 13 (8.6) | 19 (12.2) | 6 (20.0) | 0.169 | 0.113 |
| CTO | 5 (3.3) | 5 (3.2) | 2 (6.7) | 0.629 | 0.336 |
| Bifurcation | 26 (17.1) | 28 (18.1) | 4 (13.3) | 0.820 | 0.556 |
| Before procedure | |||||
| Lesion length, mm | 14.5±7.8 | 14.1±7.6 | 14.6±5.5 | 0.866 | 0.844 |
| RVD, mm | 2.32±0.52 | 2.32±0.55 | 2.49±0.61 | 0.261 | 0.101 |
| MLD, mm | 0.70±0.46 | 0.70±0.51 | 0.54±0.40 | 0.234 | 0.088 |
| %DS, % | 69.7±17.9 | 70.6±20.1 | 77.5±15.2 | 0.047 | 0.039 |
| After procedure | |||||
| RVD, mm | 2.43±0.48 | 2.37±0.50 | 2.53±0.63 | 0.175 | 0.165 |
| MLD, mm | 1.96±0.43 | 1.84±0.39 | 1.94±0.51 | 0.032 | 0.536 |
| %DS, % | 19.5±8.07 | 21.7±6.9 | 23.0±6.6 | 0.009 | 0.088 |
| Dissection (No/A/B/C) | 120/7/21/4 | 131/6/17/1 | 28/1/1/0 | 0.437 | 0.405 |
| Follow-up | |||||
| Follow-up duration, days | 215±125 | 214±115 | 220±116 | 0.956 | 0.776 |
| RVD, mm | 2.38±0.50 | 2.53±0.47 | 2.56±0.68 | 0.014 | 0.271 |
| MLD, mm | 1.71±0.43 | 2.07±0.41 | 1.05±0.37 | <0.001 | <0.001 |
| %DS, % | 27.6±11.2 | 18.0±9.7 | 59.6±7.5 | <0.001 | <0.001 |
| LLL | 0.25±0.22 | −0.24±0.20 | 0.90±0.25 | <0.001 | <0.001 |
| Dissection (No/A/B/C) | 151/0/1/0 | 155/0/0/0 | 30/0/0/0 | 0.543 | 0.754 |
| DCB size | |||||
| DCB diameter, mm | 2.70±0.47 | 2.67±0.42 | 2.84±0.50 | 0.165 | 0.074 |
| Balloon-to-artery ratio | 1.19±0.21 | 1.22±0.64 | 1.17±0.19 | 0.572 | 0.636 |
| DCB length, mm | 19.4±5.3 | 20.1±9.3 | 19.8±5.3 | 0.762 | 0.949 |
| Maximum inflation pressure, atm | 7.42±2.13 | 7.72±2.45 | 7.53±3.00 | 0.536 | 0.930 |
| Duration of inflation, s | 53.2±12.6 | 55.9±9.1 | 55.5±16.0 | 0.121 | 0.686 |
| Predilatation balloon | 0.434 | 0.154 | |||
| Conventional balloon | 22 (14.5) | 21 (13.5) | 1 (3.3) | ||
| Scoring/cutting balloon | 126 (82.9) | 130 (83.9) | 29 (96.7) | ||
| High pressure balloon | 4 (2.6) | 4 (2.6) | 0 (0) | ||
| Balloon diameter, mm | 2.59±0.48 | 2.61±0.45 | 2.80±0.53 | 0.084 | 0.027 |
| Balloon-to-artery ratio | 1.15±0.23 | 1.19±0.63 | 1.15±0.17 | 0.690 | 0.808 |
| Balloon length, mm | 12.8±2.0 | 12.8±2.2 | 12.8±2.6 | 0.991 | 0.947 |
| Maximum inflation pressure, atm | 11.9±4.7 | 11.6±4.3 | 11.3±4.6 | 0.751 | 0.608 |
| Rotablator | 3 (2.0) | 1 (0.6) | 1 (3.3) | 0.428 | 0.380 |
| DCA | 1 (0.7) | 0 (0) | 0 (0) | 0.543 | 0.754 |
| Excimer laser | 3 (2.0) | 9 (5.8) | 2 (6.7) | 0.187 | 0.470 |
| Intracoronary imaging-guided PCI | 141 (92.8) | 142 (91.6) | 28 (93.3) | 0.908 | 0.822 |
| Values are mean±standard deviation or n (%). %DS: percentage diameter stenosis; AHA: American Heart Association; CTO: chronic total occlusion; DCA: directional coronary atherectomy; DCB: drug-coated balloon; LAD: left anterior descending artery; LCx: left circumflex artery; LLL: late lumen loss; LMCA: left main coronary artery; MLD: minimal lumen diameter; PCI: percutaneous coronary intervention; RCA: right coronary artery; RVD: reference vessel diameter | |||||
Discussion
This is the first report on the classification of chronic-phase angiographic patterns after DCB angioplasty for de novo lesions. The main findings of our study were as follows: (1) 46.0% of lesions presented late lumen enlargement (LLE), (2) half of the lesions with restenosis patterns presented a focal pattern of restenosis, and (3) plaque cavities were the most frequently observed additional finding.
LUMEN ENLARGEMENT
Luminal increase during the chronic phase is a specific feature of DCB angioplasty, referred to as LLE. Previous studies have shown that the rate of LLE ranges from 50-70%56. Similarly, in this study, 46.0% of the lesions presented with late lumen enlargement. We have previously reported that the mechanisms of LLE include positive vessel remodelling and plaque regression7. Some previous reports have shown that dissection without flow limitation after DCB angioplasty might be a predictor of LLE8.
RESTENOSIS
This study showed that the binary restenosis rate after DCB angioplasty for de novo lesions was 8.9%. Previous studies reported similar binary restenosis rates (10.5%9, 11.0%10). In this study, 56.7% of patients with restenosis patterns presented with a focal restenosis pattern. A residual %DS ≥20% after lesion preparation, DCB-to-stent ratio ≥0.91, and DCB inflation time ≥60 seconds serve as predictors of restenosis after DCB angioplasty for in-stent restenosis of DES11. Although severe dissection and imaging device use have been reported as predictors of restenosis after DCB angioplasty for de novo coronary lesions12, the causes for this remain unclear.
ANEURYSM
Coronary artery aneurysms following DCB angioplasty are rare. No aneurysms were observed in the present study. Kleber et al reported that they found aneurysms after DCB intervention with an incidence of 0.8%13. The mechanism of this phenomenon after paclitaxel-coated balloon angioplasty without stenting was suspected to be vessel enlargement due to the toxic effects of the drug and the influence of any dissection.
BALLOON-EDGE RESTENOSIS
Previous studies have reported that the predictors of stent edge restenosis are a large hinge angle, residual plaque burden, lipidic plaque, and minimal lumen area in the stent-edge segment1415. Although stent-edge struts may cause mechanical stimulation of the vessel wall, leading to continuous injury and/or inflammation, DCB angioplasty can avoid the need for an implantation of a permanent metallic scaffold. The mechanism of balloon-edge restenosis after DCB angioplasty is suspected to be a predictor of geographical miss. The longitudinal geographic miss, in which the DCB does not fully cover the injured lesions by predilatation, could lead to insufficient drug delivery and failure to prevent neointimal proliferation.
PLAQUE CAVITY
Plaque cavities were the most frequently observed additional finding in the present study. On angiography, the plaque cavity appeared as an ulcer with overhanging margins (Figure 3). Although an ulcer might result from the rupture of atherosclerotic plaques in most cases, the present study showed that the plaque cavity was found not only in patients with acute coronary syndrome but also in those with stable angina. In this study, 8.0% of patients had plaque cavities on follow-up angiography. We previously reported that drug-induced plaque regression may contribute to chronic luminal enlargement7. It was suspected that the “cast-off shell” of plaque is one of the mechanisms of the plaque cavity (Figure 3C’). In this case, the normal vascular structure was maintained, and the plaque volume was reduced. Most of the abnormal dilatated findings on the follow-up angiogram after DCB angioplasty might be due to plaque cavities rather than true aneurysms.
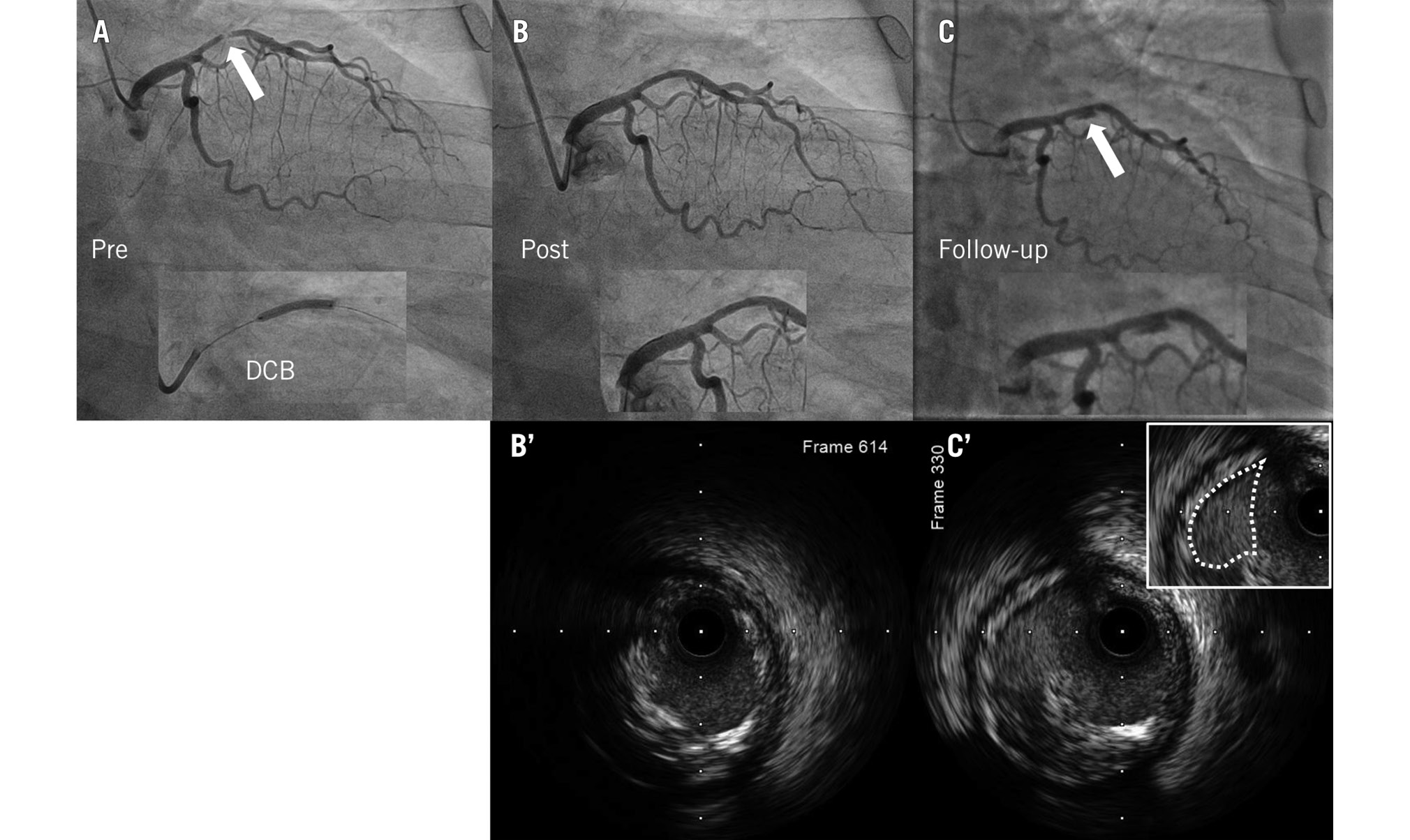
Figure 3. A representative case with a class I (non-restenosis) and type C (plaque cavity) lesion. A) Preprocedure; (B) post-DCB treatment; (C) follow-up angiographic images showing the plaque cavity (arrow); (B’) IVUS image post-DCB treatment; (C’) IVUS image at follow-up showing the plaque cavity (dotted line). DCB: drug-coated balloon; IVUS: intravascular ultrasound
RESIDUAL DISSECTION
The international DCB consensus group recommended that the absence of flow-limiting dissection is a factor for acceptable angiographic results after lesion preparation. A previous study reported that more than 90% of dissections after DCB angioplasty healed completely despite the initial severity, and there were no new or worse dissections16. The present study demonstrated a residual dissection rate of 0.3%.
Limitations
This study had some limitations. First, this was a retrospective study, and a selection bias may have occurred. Second, because this was a single-centre study, the number of patients was small. Third, angiogram at follow-up was not mandatory; therefore, the rate of angiographic follow-up was approximately 50%. These data might be insufficient, and silent ischaemia could have been underestimated. Fourth, the association between the findings of intracoronary imaging devices and angiographic classification was not assessed in this study. The sample size of the restenosis group was small. Further study involving follow-up intracoronary evaluation is needed to reveal any association.
Conclusions
This report demonstrates for the first time the angiographic classification after DCB angioplasty for de novo coronary lesions. Restenosis patterns were seen in 8.9% of lesions, and half of the restenosis patterns presented a focal restenosis pattern. Late lumen enlargement was observed in 46% of the treated lesions; however, it is still unclear whether angiographic classification after DCB angioplasty has an impact on clinical events. We expect the impact of this classification on clinical outcomes to be revealed in the future.
Impact on daily practice
This report demonstrates for the first time the angiographic classification after DCB angioplasty for de novo coronary lesions. Late lumen enlargement was observed in 46% of the treated lesions. Half of the restenosis patterns presented a focal restenosis pattern. In the future, it is expected that the impact of this classification on clinical outcomes will be demonstrated.
Conflict of interest statement
The authors have no conflicts of interest to declare.

