Ippei Watanabe*, MD, PhD; Daiga Saito, MD, PhD; Ryota Noike, MD; Takayuki Yabe, MD, PhD; Ryo Okubo, MD, PhD; Rine Nakanishi, MD, PhD; Hideo Amano, MD, PhD; Mikihito Toda, MD, PhD; Takanori Ikeda, MD, PhD
AsiaIntervention 2019;5:134-140, DOI: 10.4244/AIJ-D-18-00051
Department of Cardiovascular Medicine, Department of Internal Medicine, Toho University Faculty of Medicine, Tokyo, Japan
I. Watanabe and D. Saito contributed equally to this study.
Abstract
Aims: This study aimed to evaluate the clinical significance of measuring left ventricular end-diastolic pressure (LVEDP) in patients with ST-segment elevation myocardial infarction (STEMI).
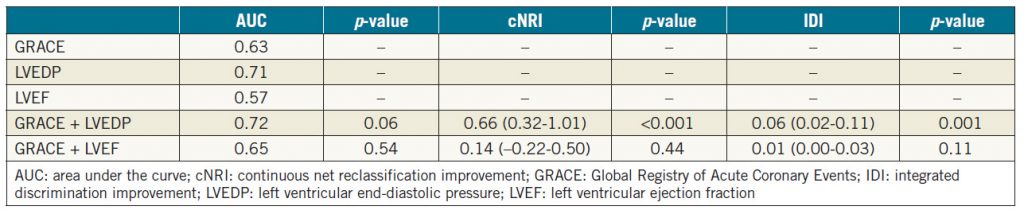
Methods and results: We retrospectively analysed clinical data from 277 patients with STEMI between October 2006 and June 2014. LVEDP and left ventricular ejection fraction (LVEF) were perioperatively measured during percutaneous coronary intervention (PCI). The primary endpoint was a major adverse cardiac event (MACE) such as cardiac death, non-fatal myocardial infarction, or hospitalisation due to heart failure during the observation period. The independent predictors were identified by Cox proportional hazards regression analysis. Continuous net reclassification improvement (cNRI) and integrated discrimination improvement (IDI) were conducted to assess the incremental prognostic value of adding cardiovascular parameters, including LVEDP, to the Global Registry of Acute Coronary Events (GRACE) score. The mean follow-up period was 44±31 months. A MACE occurred in 33 patients (12.0%). In the Cox proportional hazards regression model, after adjusting for confounding factors, LVEDP was an independent predictor of a MACE (hazard ratio [HR] 1.11, 95% confidence interval [CI]: 1.06-1.17, p<0.001). In addition, the predictive value of the GRACE score for a MACE was significantly improved by LVEDP (NRI 0.66, 95% CI: 0.32-1.01, p<0.001; IDI 0.06, 95% CI: 0.02-0.11, p=0.001), but not by LVEF (NRI 0.14, 95% CI: -0.22-0.50, p=0.44; IDI 0.01, 95% CI: 0.00-0.03, p=0.11).
Conclusions: The results of this study demonstrated that evaluating LVEDP provides an additive prognostic value over conventional risks estimated by the GRACE score among STEMI patients.
Abbreviations
GRACE: Global Registry of Acute Coronary Events
IABP: intra-aortic balloon pump
IDI: integrated discrimination improvement
LVEDP: left ventricular end-diastolic pressure
LVEDV: left ventricular end-diastolic volume
LVEF: left ventricular ejection fraction
MACE: major adverse cardiac event
NRI: net reclassification improvement
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
The incidence of ST-segment elevation myocardial infarction (STEMI) remains a leading cause of morbidity and mortality in patients with atherosclerotic risk factors. Myocardial ischaemia (MI) after STEMI initiates both systolic and diastolic myocardial dysfunction with subsequent advanced left ventricular (LV) remodelling1,2. Therefore, the development of more physiologically integrative methods for predicting global LV function during the acute phase of STEMI may be required for a better prognosis. LV end-diastolic pressure (LVEDP) is easily obtained from catheterisation during the follow-up of patients with STEMI. Currently, there are accumulating data on LVEDP in predicting outcomes in patients with MI3-5. Risk stratification using clinical markers or parameters such as the Global Registry of Acute Coronary Events (GRACE) score has been widely used to predict clinical outcomes after STEMI6,7. However, the GRACE score lacks haemodynamic information defining LV systolic and diastolic function. Therefore, this study investigated the additive prognostic value of LVEDP over the GRACE score in patients with STEMI undergoing successful percutaneous coronary intervention (PCI).
Methods
STUDY POPULATION
This retrospective study analysed data from 277 consecutive patients who underwent PCI for STEMI at Toho University Omori Medical Center (Tokyo, Japan) between October 2006 and June 2014. Patients were included if they had STEMI with characteristic chest pain within 12 hours before hospital admission. All patients underwent successful PCI with subsequent left ventriculography (LVG) to measure LVEDP and LV ejection fraction (LVEF). STEMI was diagnosed by electrocardiography as (i) an ST elevation of ≥2 mm either in two contiguous anterior-lateral leads or in inferior leads, or (ii) a new left bundle branch block with concordant ST elevation of 1 mm8. Patients who lacked LVG data and had a Thrombolysis In Myocardial Infarction (TIMI) flow grade of <3 after PCI were excluded. This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the relevant ethics committee at Toho University Faculty of Medicine (No. M16259). Baseline clinical information was obtained from medical records. Cardiovascular risk factors including diabetes, dyslipidaemia, hypertension, and current smoking were defined in accordance with the accepted criteria9-11. Baseline laboratory data and information on blood pressure and heart rate were collected at admission. Troponin I and creatinine kinase myocardial band (CKMB) were measured at least twice a day, until peak values were recorded. For each patient, the GRACE risk score was calculated using eight specific variables collected upon admission as reported (http://www.gracescore.org/website/webversion.aspx).
The primary endpoint was a major adverse cardiac event (MACE), including cardiac death, non-fatal myocardial infarction, and heart failure requiring hospitalisation, during the observation period.
INVASIVE CORONARY ANGIOGRAPHY PROTOCOL
Primary PCI was performed according to standard methods8. Patients who received a diagnosis of STEMI were treated with 100 mg aspirin and either 150 mg clopidogrel or 200 mg ticlopidine before catheterisation. There was no case of thrombolysis during PCI. Procedural success was defined as a successful guidewire and balloon crossing with residual stenosis >50% and TIMI flow grade ≥3 after coronary stenting. Measurements of LVEDP and LVEF during LVG were performed as described12.
STATISTICAL ANALYSIS
Data were analysed with the Statistical Package for EZR for Windows, Version 1.35 (Saitama Medical Center, Japan)13. Continuous variables are expressed as the mean±standard deviation. The Kolmogorov-Smirnov test was applied to test for normal distribution. Continuous variables were compared using the Student’s t-test. Demographics, traditional risk factors, and clinical outcomes were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate, for categorical data, and were expressed as percentages. A Kaplan-Meier analysis was performed to calculate the unadjusted MACE rate according to the median value of LVEDP. Cox proportional hazards regression analysis was used to identify independent predictors of MACE during the observation period. The multivariate model was built by backward stepwise variable selection, with entry and exit criteria set at p<0.05. We used the area under the curve (AUC) by receiver operating characteristic for the prediction of MACE to assess the added value of LVEDP or LVEF over assessment of the GRACE score. Continuous net reclassification improvement (cNRI) and integrated discrimination improvement (IDI) were also used to investigate whether LVEDP or LVEF reclassified patients with respect to MACE risk relative to their GRACE score.
Results
PATIENT CHARACTERISTICS AND INCIDENCE OF MACE
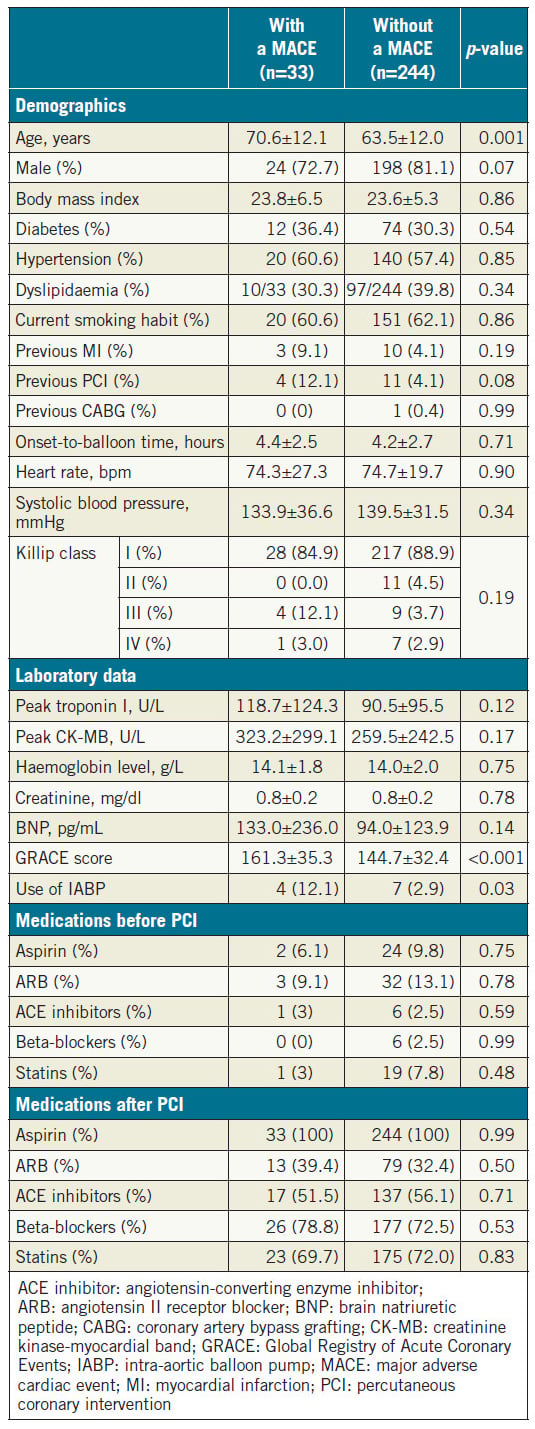
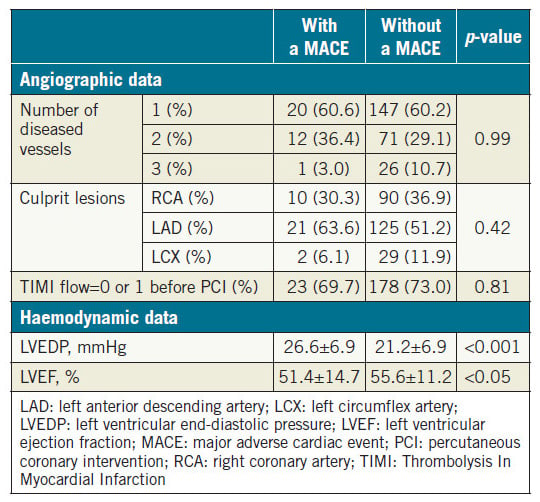
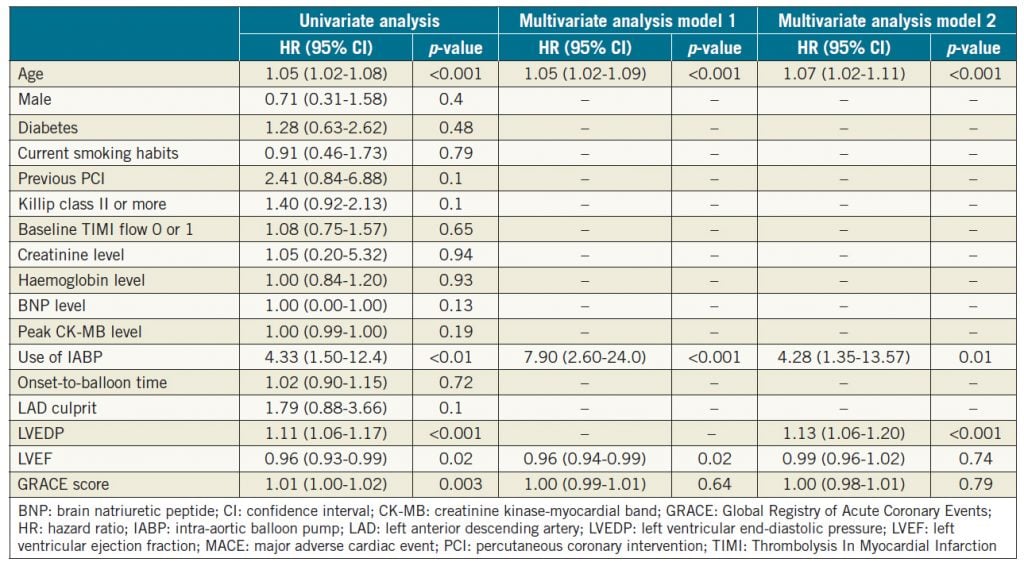
We evaluated 277 patients hospitalised due to confirmed STEMI. During the mean follow-up period of 44±31 months, 33 patients (12.0%) developed a MACE (Table 1). Patients with a MACE were older than those without a MACE. The GRACE score was significantly higher and the use of an intra-aortic balloon pump (IABP) was more frequent in patients with a MACE relative to those who did not experience such an event. There was a tendency towards statistical significance in gender and prior PCI rates between the two groups. There was no significant difference in prescribed medications including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, and statins after STEMI between the two groups. Table 2 shows the angiographic and haemodynamic data during catheterisation. There was no significant difference in the number of diseased vessels and the location of culprit lesions found with coronary angiography between the two groups. In contrast, LVEDP was significantly higher and LVEF was significantly lower in patients with a MACE. Among the MACE components, there were 13 cardiac deaths (5.0%), 10 incidents of non-fatal myocardial infarction (3.6%), and 10 hospitalisations due to heart failure (3.6%).
ASSOCIATION BETWEEN HIGHER LVEDP AND THE INCIDENCE OF MACE
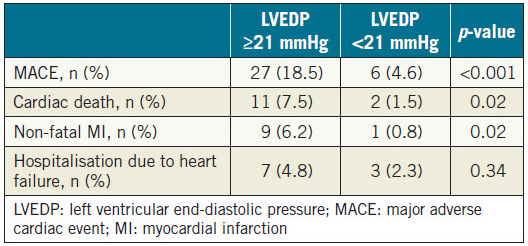
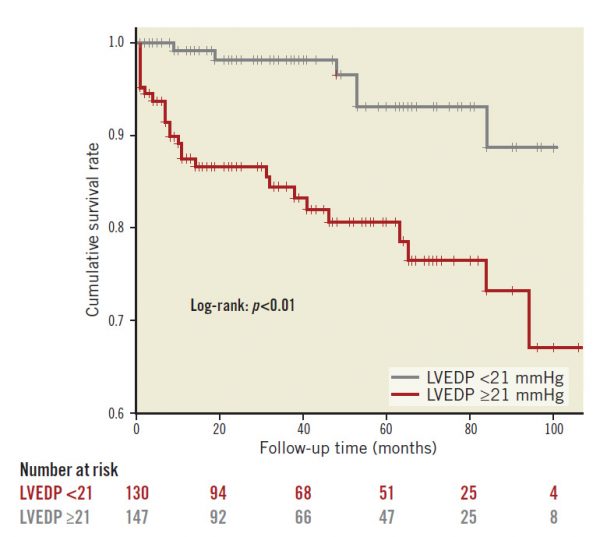
Patients were divided into two groups according to the median value of LVEDP (21 mmHg). As shown in Table 3, patients with LVEDP ≥21 mmHg had higher incidences of cardiac death, nonfatal myocardial infarction and MACE, as compared with patients with LVEDP <21 mmHg. Also, on Kaplan-Meier analysis, patients with LVEDP ≥21 mmHg showed higher rates of MACE incidence (Figure 1).
Table 1. Baseline characteristics of patients with and without a MACE.
Table 2. Catheterisation analysis of patients with and without a MACE.
Table 3. Incidence of MACE components according to the median value of LVEDP (21 mmHg).
Figure 1. Kaplan-Meier curves for MACE according to the median value of LVEDP (21 mmHg).
INDEPENDENT PREDICTORS OF MACE
Age, use of IABP, LVEDP, LVEF, and GRACE score were applied to the Cox proportional hazards regression model to identify independent predictors of a MACE. In Table 4, after adjustment by these variables without LVEDP, age, use of IABP and LVEDP were associated with the incidence of a MACE (model 1). In the full adjusted model, LVEDP, age, and the use of IABP were found to be associated with the incidence of a MACE.
INCREMENTAL PROGNOSTIC VALUE OF LVEDP OVER THE GRACE SCORE
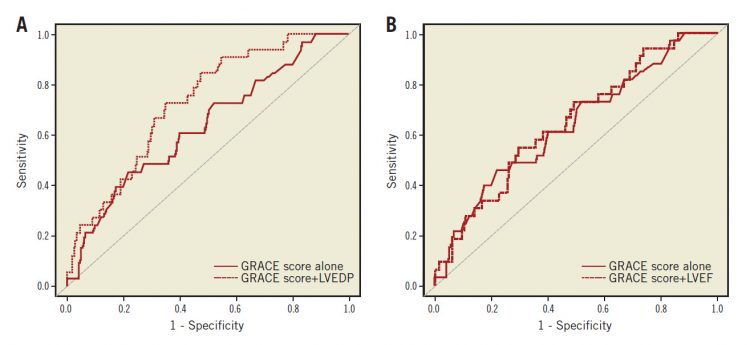
For the incidence of MACE, the incremental predictive value of LVEDP and LVEF over the GRACE score was evaluated. As shown in Figure 2A and Figure 2B, including the LVEF data with the GRACE score did not improve the area under the curve (AUC) for the GRACE score alone (p=0.54), but there was a tendency towards statistical significance for the LVEDP to increase the AUC over the GRACE score alone (p=0.06). In addition, we assessed the additive predictive value of the LVEDP or LVEF in combination with the GRACE score by cNRI and IDI. As shown in Table 5, the addition of LVEDP successfully recategorised patients based on the GRACE score alone, but the addition of LVEF did not.
Figure 2. The predictive value of LVEDP and LVEF by ROC curve analysis for MACE incidence. A) Comparison of the AUC to predict MACE between GRACE score and GRACE score + LVEDP. B) Comparison of the AUC to predict MACE between GRACE score and GRACE score + LVEF
Discussion
We evaluated the possibility of using LVEDP for the early risk stratification of patients with STEMI who were successfully treated by PCI. LVEDP was significantly higher in patients who developed a MACE. In addition, LVEDP provided an incremental prognostic value over the GRACE risk score according to reclassification analyses, but LVEF did not. This study demonstrated that the LVEDP was superior to other common clinical factors such as infarct size and LVEF in predicting long-term outcomes after STEMI. Our negative impact of LVEF at admission was consistent with the data by Dutcher et al7. Of note, as was shown in the previous study, we also found that LVEF is an independent predictor of a MACE in multivariate analysis14. However, this impact was eliminated when adjusted by LVEDP. These interesting findings may be due to the relationship between an increase in LVEDP and subsequent LV remodelling. As described in the Frank-Starling law, compensatory increases in LV end-diastolic volume (LVEDV) and LVEDP maintain stroke volume during severe LV dysfunction after STEMI15. In those cases, the renin-angiotensin system and sympathetic nervous system are highly activated with inducible monotype hypertrophy and compensatory LV dilation in the long term16-18. According to a study by Garber et al19, LVEDV at baseline is an independent predictor of LV remodelling after STEMI. The ventricular dilation and wall thinning that result from infarct zone expansion reportedly increase LVEDV during the early phase of MI. Interestingly, we previously reported that increasing LVEDP is associated with LV dilation during the acute phase after STEMI, showing that both LV end-systolic volume index (LVESVI) and LV end-diastolic volume index are significantly higher in patients with higher LVEDP5. In addition, we also found that there is a relationship between higher LVEDP and subsequent progression of LV remodelling after STEMI, showing that LVESVI after PCI remains higher during long-term follow-up in patients with higher LVEDP, irrespective of baseline LVEF. Through these processes, the higher LVEDP may influence LV function and long-term clinical outcomes after STEMI. Therefore, this invasive but simple method to assess global LV function and systemic haemodynamics may affect current early risk stratification after STEMI. We found that the GRACE score was not a significant factor in our multivariate analysis, suggesting that it is basically more suitable for assessing the short-term outcome after STEMI, whereas limited studies have demonstrated that it is also a preferred scoring system for risk stratification in the long term20,21. Of importance, the GRACE score does not provide haemodynamic information (i.e., LVEDP or LVEF); very little has been investigated about the additive impact of LVEDP to risk stratification using prognostic factors over clinical risk scores including the GRACE score. This study demonstrated that its inclusion resulted in a tendency to increase the AUC relative to that with the GRACE score alone to predict MACE incidence. In addition, by adding the LVEDP information to the GRACE score, 66% of patients were successfully recategorised. Interestingly, these effects were not observed by inclusion of LVEF. Our results are similar to those in a study by Abu-Assi et al, which showed that the addition of LVEF did not provide incremental prognostic information to the GRACE score in patients with acute coronary syndrome22. The authors found that there was high collinearity between the GRACE score and LVEF, suggesting that the GRACE score predicts prognosis effectively irrespective of LVEF. These results were consistent with a previous study in which a higher LVEDP was found to have comparable prognostic value with risk scores including the GRACE risk score23. In the light of these data, it is worth emphasising the periprocedural assessment of LVEDP during PCI in patients with STEMI.
Table 4. Cox proportional hazards regression model to predict a MACE.
Table 5. Reclassification analysis of patients based on the GRACE score alone and the GRACE score with the LVEDP or LVEF.
Limitations
This study had the following limitations. This was a single-centre, observational cohort study of a relatively small population. In this retrospective setting, a variability of follow-up duration according to the different enrolment timing might have affected the results. In fact, most of the patients during 2011 and 2014 were followed for less than the mean follow-up period of 44 months (97 out of 110). In addition, because we possibly excluded those with very low LVEF due to intolerability to undergo LVG, the subsequent prognostic impact of LVEF might be underestimated. Thus, a prospective large-scale multicentre clinical setting is needed for further confirmation. Of importance, as was shown in our previous reports5, the combined assessment of LVEDP and LVEF at admission was a useful prognostic parameter after STEMI. Therefore, the pathophysiological mechanism of LV remodelling after STEMI may be clarified by serial haemodynamic assessment including LVEDP and LVEF in the larger sample size. In addition, lower LVEF and larger infarct size were not associated with worse outcome in the current study, because patients with cardiac shock and higher peak troponin I levels were excluded from this study due to the lack of data on LVG. In addition, the optimal timing of LVEDP measurements remains unclear. LVEDP measurements at different time points may be more informative for treatment strategy and predicting prognosis. Finally, the relationship between increasing LVEDP and LV remodelling needs further assessment. For example, imaging modalities such as cardiac magnetic resonance may be less invasive and more useful for assessing the progression of LV remodelling.
Conclusions
Including LVEDP measurements provides additional information to the GRACE risk score for assessing the risk of a MACE in patients with STEMI. Further prospective approaches should be investigated to determine if LVEDP reduction guides care after MI.
Impact on daily practice
The assessment of LVEDP guides care after STEMI.
Funding
This research was supported in part by Grants-in-Aid (Nos. 15K09103 and 16K01433 to T.I.) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. De Luca L, Sardella G, Davidson CJ, De Persio G, Beraldi M, Tommasone T, Mancone M, Nguyen BL, Agati L, Gheorghiade M, Fedele F. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST elevation myocardial infarction. Heart. 2006;92: 951-7.
2. Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Watkins S, Hood S, Davie A, Mahrous A, Sattar N, Welsh P, Tzemos N, Radjenovic A, Ford I, Oldroyd KG, Berry C. Pathophysiology of LV Remodeling in Survivors of STEMI: Inflammation, Remote Myocardium, and Prognosis. JACC Cardiovasc Imaging. 2015;8:779-89.
3. Planer D, Mehran R, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Reyes SL, Stone GW. Prognostic utility of left ventricular end-diastolic pressure in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;108:1068-74.
4. Bagai A, Armstrong PW, Stebbins A, Mahaffey KW, Hochman JS, Weaver WD, Patel MR, Granger CB, Lopes RD. Prognostic implications of left ventricular end-diastolic pressure during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: Findings from the Assessment of Pexelizumab in Acute Myocardial Infarction study. Am Heart J. 2013;166:913-9.
5. Saito D, Nakanishi R, Watanabe I, Yabe T, Okubo R, Amano H, Toda M, Ikeda T. Combined assessment of left ventricular end-diastolic pressure and ejection fraction by left ventriculography predicts long-term outcomes of patients with ST-segment elevation myocardial infarction. Heart Vessels. 2018;33:453-61.
6. Parenica J, Kala P, Pavkova MG, Tomandl J, Spinar J, Littnerova S, Jarkovsky J, Mebazaa A, Tomandlova M, Dastych M, Gottwaldova J, Gayat E. Natriuretic peptides, nitrite/nitrate and superoxide dismutase have additional value on top of the GRACE score in prediction of one-year mortality and rehospitalisation for heart failure in STEMI patients – Multiple biomarkers prospective cohort study. Int J Cardiol. 2016;211:96-104.
7. Dutcher JR, Kahn J, Grines C, Franklin B. Comparison of left ventricular ejection fraction and exercise capacity as predictors of two- and five-year mortality following acute myocardial infarction. Am J Cardiol. 2007;99:436-41.
8. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez- Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-619.
9. Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, Mas JL, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic eventsstudy design. Am Heart J. 2006;151:786.
10. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-421.
11. Parikh NI, Gona P, Larson MG, Fox CS, Benjamin EJ, Murabito JM, O’Donnell CJ, Vasan RS, Levy D. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute’s Framingham Heart study. Circulation. 2009;119:1203-10.
12. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O’Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE Jr, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis- Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135-47.
13. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-8.
14. Daneault B, Généreux P, Kirtane AJ, Witzenbichler B, Guagliumi G, Paradis JM, Fahy MP, Mehran R, Stone GW. Comparison of Three-year outcomes after primary percutaneous coronary intervention in patients with left ventricular ejection fraction <40% versus ≥40% (from the HORIZONS-AMI trial). Am J Cardiol. 2013;111:12-20.
15. Lew WY, Ban-Hayashi E. Mechanisms of improving regional and global ventricular function by preload alterations during acute ischemia in the canine left ventricle. Circulation. 1985;72: 1125-34.
16. Parashar A, Agarwal S, Krishnaswamy A, Garg A, Poddar KL, Sud K, Ellis S, Tuzcu EM, Kapadia SR. Renin-Angiotensin System Antagonists in Patients Without Left Ventricular Dysfunction After Percutaneous Intervention for ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2015;116:508-14.
17. Park H, Kim HK, Jeong MH, Cho JY, Lee KH, Sim DS, Yoon NS, Yoon HJ, Hong YJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kim YJ, Cho MC, Kim CJ; Korea Acute Myocardial Infarction Registry Investigators. Clinical impacts of inhibition of renin-angiotensin system in patients with acute ST-segment elevation myocardial infarction who underwent successful late percutaneous coronary intervention. J Cardiol. 2017;69: 216-21.
18. Kasama S, Toyama T, Sumino H, Kumakura H, Takayama Y, Minami K, Ichikawa S, Matsumoto N, Sato Y, Kurabayashi M. Effects of spironolactone on cardiac sympathetic nerve activity and left ventricular remodelling after reperfusion therapy in patients with first ST-segment elevation myocardial infarction. Heart. 2011;97:817-22.
19. Garber L, McAndrew TC, Chung ES, Stancak B, Svendsen JH, Monteiro J, Fischer TM, Kueffer F, Ryan T, Bax J, Leon AR, Stone GW. Predictors of Left Ventricular Remodeling After Myocardial Infarction in Patients With a Patent Infarct Related Coronary Artery After Percutaneous Coronary Intervention (from the Post-Myocardial Infarction Remodeling Prevention Therapy [PRomPT] Trial). Am J Cardiol. 2018;121:1293-8.
20. Littnerova S, Kala P, Jarkovsky J, Kubkova L, Prymusova K, Kubena P, Tesak M, Toman O, Poloczek M, Spinar J, Dusek L, Parenica J. GRACE Score among Six Risk Scoring Systems (CADILLAC, PAMI, TIMI, Dynamic TIMI, Zwolle) Demonstrated the Best Predictive Value for Prediction of Long-Term Mortality in Patients with ST-Elevation Myocardial Infarction. PloS One. 2015;10:e0123215.
21. Kozieradzka A, Kaminski KA, Maciorkowska D, Olszewska M, Dobrzycki S, Nowak K, Kralisz P, Prokopczuk P, Musial WJ. GRACE, TIMI, Zwolle and CADILLAC risk scores- -do they predict 5-year outcomes after ST-elevation myocardial infarction treated invasively? Int J Cardiol. 2011;148:70-5.
22. Abu-Assi E, Ferreira-Gonzalez I, Ribera A, Marsal JR, Cascant P, Heras M, Bueno H, Sanchez PL, Aros F, Marrugat J, Garcia-Dorado D, Pena-Gil C, Gonzalez-Juanatey JR, Permanyer- Miralda G. “Do GRACE (Global Registry of Acute Coronary events) risk scores still maintain their performance for predicting mortality in the era of contemporary management of acute coronary syndromes?”. Am Heart J. 2010;160:826-34.
23. Sola M, Venkatesh K, Caughey M, Rayson R, Dai X, Stouffer GA, Yeung M. Ratio of systolic blood pressure to left ventricular end-diastolic pressure at the time of primary percutaneous coronary intervention predicts in-hospital mortality in patients with ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;90:389-95.
To download, please click below.