Ayman J. Hammoudeh1*, MD, FACC; Nahla Al-Bayyari2, BcPH, MPH, PhD; Omar Obeidat3, MD, FACC; Eyas N. Al-Mousa1, MD, FACC; Ramzi A. Tabbalat4, MD, FACC; Imad A. Alhaddad5, MD, FACP, FACC
AsiaIntervention 2019;5:18-26, DOI: 10.4244/AIJ-D-17-00049
1. Cardiology Department, Istishari Hospital, Amman, Jordan; 2. Department of Nutrition and Food Technology, Faculty of Al-Huson University College, Al-Balqa Applied University, Al-Salt, Jordan; 3. Division of Cardiology, Department of Internal Medicine, Jordan University Hospital, Amman, Jordan; 4. Department of Cardiology, Khalidi Medical Center, Amman, Jordan; 5. Division of Cardiology, Department of Internal Medicine, Jordan Hospital Medical Center, Amman, Jordan
Abstract
Aims: The aim of this study was to evaluate the impact on prognosis of renal impairment (RI) in Middle Eastern patients after percutaneous coronary intervention (PCI).
Methods and results: PCI patients (N=2,426) were divided into three groups according to the estimated glomerular filtration rate (eGFR, ml/min/1.73 m2): normal renal function (eGFR ≥90), mild RI (eGFR 60-89), or moderate to severe RI (eGFR <60). Mean age of participants was 56±11 years. Normal renal function was present in 41.6%, mild RI in 44.2%, and moderate to severe RI in 14.2%. Patients with moderate to severe RI were older and had higher prevalence of hypertension and diabetes mellitus compared with other patients (p≤0.002). At one year, patients with moderate to severe RI had a higher incidence of cardiac mortality (3.78%) compared with patients with mild (1.77%) or no RI (1.49%), p=0.03. In multivariate analysis, moderate to severe RI was associated with higher one-year cardiac mortality compared to mild or no RI (odds ratio=3.7; 95% CI: 2.8-5.0, p=0.001).
Conclusions: Impaired renal function was present in about six out of 10 Middle Eastern patients undergoing PCI. Moderate to severe RI carries a higher risk of cardiac mortality at one year compared with mild or no RI.
Abbreviations
ACS: acute coronary syndrome
BMI: body mass index
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CI: confidence interval
CVD: cardiovascular disease
DM: diabetes mellitus
eGFR: estimated glomerular filtration rate Hct haematocrit
JoPCR1: first Jordanian percutaneous coronary intervention registry
MDRD: Modification of Diet in Renal Disease equation
NSTE-ACS: non-ST-segment elevation acute coronary syndrome
NSTEMI: non-ST-segment elevation myocardial infarction
OR: odds ratio
PCI: percutaneous coronary intervention
RBC: red blood cell
RI: renal impairment
SD: standard deviation
STEMI: ST-segment elevation myocardial infarction
UA: unstable angina
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the Middle East1,2. The number of patients who undergo percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) and stable coronary disease in this region is increasing3,4. Impaired renal function is a known risk factor for CVD, has a significant prevalence among patients who are admitted with ACS5,6, and is associated with an increased risk of cardiovascular events including death and major bleeding during the index hospitalisation and long-term follow-up7,8. Furthermore, patients who have renal impairment (RI) are frequently excluded from cardiovascular outcome trials9.
The incidence of impaired renal function and its impact on short- and long-term outcome have not been evaluated in an exclusive PCI population in the Middle East. Only a few large regional registries have addressed cardiovascular outcome among patients admitted with ACS in relation to the severity of RI10,11. Of those studies, two evaluated the incidence of renal dysfunction and its impact on the in-hospital outcome in patients admitted with ACS and ST-segment elevation myocardial infarction (STEMI)12,13. Major limitations of these studies include the low rate of utilisation of coronary angiography and PCI, the heterogeneous groups of enrolled patients (native citizens and South Asian workers) and the lack of evaluation of long-term outcome.
We utilised data from the prospective, multicentre first Jordanian PCI Registry (JoPCR1)14 to study in detail the prevalence of RI and its impact on the short- and long-term outcome after PCI in a Middle Eastern country. The study was designed to address cardiovascular outcome in an increasing number of patients who undergo PCI on an emergency or elective basis in this region. We aimed to evaluate the incidence of various degrees of RI in a PCI population, clinical and coronary angiographic features of patients who have RI, and the impact of RI on major adverse events during the index hospitalisation and up to one year of follow-up.
Methods
JoPCR1 is a prospective, observational, multicentre registry of consecutive patients who underwent PCI at 12 tertiary care centres in Jordan between January 2013 and February 2014. Data were recorded prospectively at hospital admission and discharge, and at one, six and 12 months after the discharge. Data were collected during out-patient clinic visits or by phone calls to patients, household relatives or primary care physicians. Baseline data included clinical, laboratory, electrocardiographic, echocardiographic, and coronary angiographic features. Severity of coronary artery disease (CAD) was categorised as single CAD (≥70% luminal narrowing of one epicardial coronary artery, except the left main, on coronary angiography), or multivessel CAD (≥70% luminal narrowing of at least two epicardial coronary arteries or ≥50% of the left main coronary artery). Single-vessel PCI refers to percutaneous intervention in one epicardial coronary artery, except the left main, regardless of the number of stents used.
Multivessel PCI refers to percutaneous intervention in two or more coronary arteries regardless of the number of stents used. Baseline serum creatinine values were measured for all patients at admission, and renal function was assessed online by the estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) equation15. Stages of RI were defined according to Chronic Kidney Disease Working Group guidelines and categorised according to the eGFR into three groups: normal renal function (≥90 mL/min/1.73 m2), mild RI (60-89 mL/min/1.73 m2), and moderate to severe RI (<60 mL/min/1.73 m2)16. Clinical, electrocardiographic, and coronary angiographic profiles, and inhospital and one-year major adverse cardiovascular events were evaluated in the three groups of patients.
All PCI procedures were performed according to current standard guidelines. Details of the PCI procedures and complications were also prospectively recorded during hospitalisation and up to one year of follow-up. The arterial access site, type and number of stents, and the use of oral and intravenous antiplatelet agents were left to the operator’s discretion. PCI was indicated for either ACS or stable coronary disease. ACS was classified as STEMI or non- ST-segment elevation ACS (NSTE-ACS) that included NSTEMI and unstable angina (UA). Stable coronary disease was defined as either chronic stable angina or silent ischaemia.
The studied adverse cardiovascular events were cardiac mortality, stent thrombosis, major bleeding events, stroke, coronary revascularisation and readmission for heart failure or ACS in the three groups. Cardiac mortality was defined as any death not attributed to a clear non-cardiac cause. Definite or probable stent thrombosis was defined according to the Academic Research Consortium definition17. Major bleeding events were defined according to the CRUSADE study classification and included intracranial haemorrhage, retroperitoneal bleeding, haematocrit (Hct) drop ≥12% from baseline, any red blood cell (RBC) transfusion when baseline Hct was ≥28%, or any RBC transfusion when baseline Hct was <28% with witnessed bleeding18. The study was approved by the Institutional Review Board of each participating hospital.
STATISTICAL ANALYSIS
Collected data were analysed using the SPSS statistical package, Version 22 (IBM Corp., Armonk, NY, USA). Descriptive statistics were compiled using means and standard deviation (SD) to describe the continuous variables. Frequencies and percentages were used to describe the categorical variables. The differences between the means of the normally distributed variables were examined using multivariate tests of the general linear model in the three groups of patients with different degrees of RI. Correlations of RI with age, gender, diabetes mellitus (DM), past history of CVD, ST-segment deviation, elevated cardiac biomarkers, multivessel CAD, and heart failure were analysed using Pearson’s two-tailed correlation for numeric variables. Frequencies and percentages for each stratum according to the participants’ eGFR were calculated and compared using the Mantel-Haenszel chi-square (χ²) and Fisher’s exact tests. A multivariable binary logistic regression analysis was performed to examine collectively the association between the cardiac deaths at one year and the significant risk factors identified from the chi-square univariate and multivariate analyses. The final multivariable logistic regression model for cardiac deaths at one year was constructed using manual stepwise forward logistic regression analysis. All reported p-values were two-tailed, and p≤0.05 was considered to be statistically significant.
Results
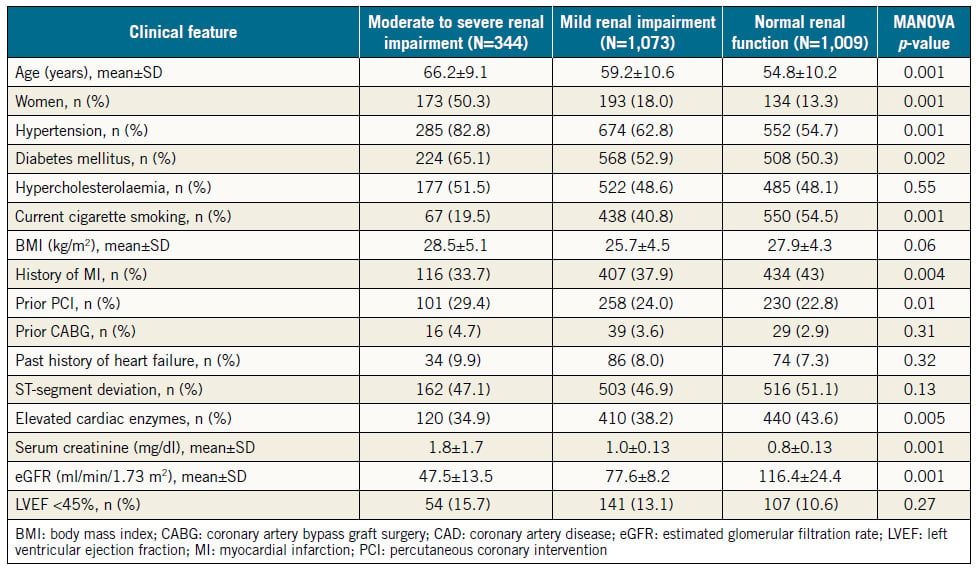
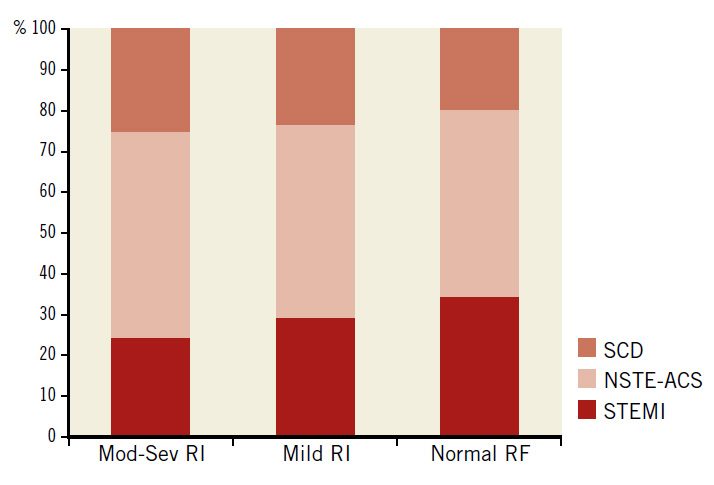
The baseline features of the consecutive patients who had PCI (N=2,426) are shown in Table 1 in three groups according to the renal function. Normal renal function was present in 1,009 (41.6%), mild RI in 1,073 (44.2%), and moderate to severe RI in 344 (14.2%) of all patients. Among patients in the latter group, 308 (12.7%) had moderate RI (eGFR 30-59 ml/min/1.73 m2), 17 (0.7%) had severe RI (eGFR 15-29 ml/min/1.73 m2), and 19 (0.8%) had very severe RI (eGFR <15 ml/min/1.73 m2). There were 26 (1.1%) haemodialysis-dependent patients. Patients who had severe or very severe RI (N=36, 1.5%) were grouped with those who had moderate RI because the validity of statistical analysis of such a small sample might be compromised. Patients with moderate to severe RI were older, more likely to be female, to have hypertension and DM, and tended to be more overweight than patients with normal renal function or mild RI. Compared with patients who had mild or no RI, those with moderate to severe RI had more stable coronary disease as an indication for PCI (27% vs. 24% vs. 20%, respectively, p=0.03), and less STEMI as an indication for PCI (23% vs. 29% vs. 34%, respectively, p=0.001) (Figure 1).
Table 1. Baseline features in three groups of patients stratified by the estimated glomerular filtration rate.
Figure 1. Indications for PCI in 2,426 PCI patients according to the degree of renal impairment. Mod-Sev RI: moderate to severe renal impairment; NSTE-ACS: non-ST-segment elevation acute coronary syndrome; RF: renal function; SCD: stable coronary disease; STEMI: ST-segment elevation myocardial infarction
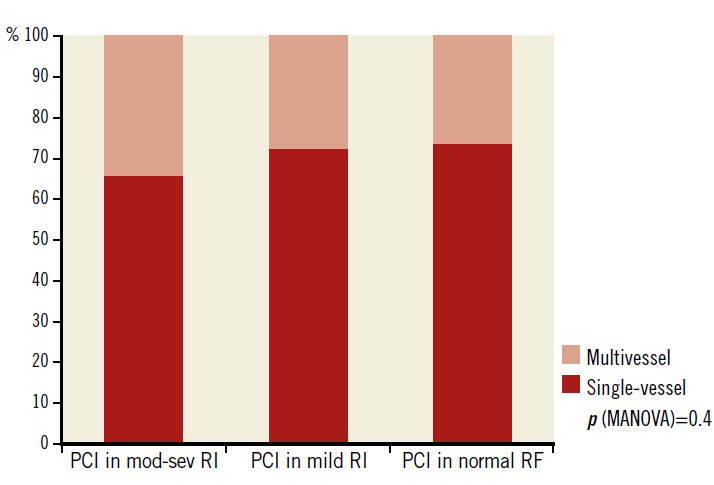
Figure 2. Single-vessel and multivessel PCI in 2,426 patients according to the degree of renal impairment. mod-sev RI: moderate to severe renal impairment; PCI: percutaneous
Patients with moderate to severe RI also had higher prevalence of multivessel CAD than the other two groups (47% vs. 42% vs. 39%, respectively, p=0.03). However, the differences in the rates of PCI for multivessel CAD in the three groups were not significant (34% vs. 28% vs. 27%, respectively, p=0.41) (Figure 2). Most of the coronary stents used (N=3,038) were drug-eluting (90%). Bare metal stents and bioresorbable scaffolds comprised 9% and 1% of all stents, respectively.
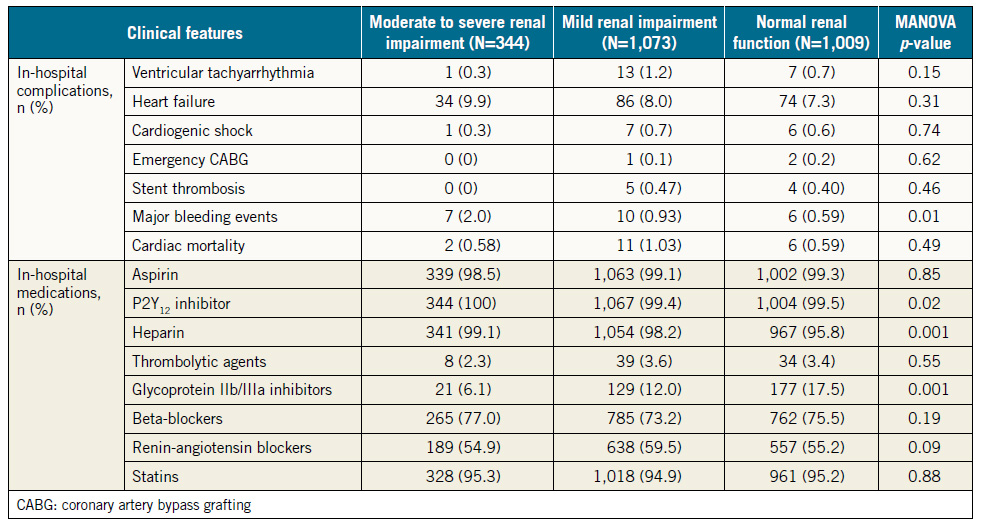
Table 2 depicts in-hospital complications and use of medications in the three groups of patients. Of seven major in-hospital complications, major bleeding was the only event that occurred in a significantly higher proportion of patients with moderate to severe RI compared with patients with normal renal function (2.0% vs. 0.6%, p=0.02). The incidence rates of other events, heart failure, cardiogenic shock, ventricular tachyarrhythmias, and emergency coronary bypass surgery were not statistically different among the three groups.
There were no differences among the three groups of patients in the rates of in-hospital use of the four classes of recommended cardiovascular medications (dual antiplatelet agents, statins, beta-blockers and renin-angiotensin blockers). Similarly, at one year, the rates of use of these four classes of medications were not different among the three groups of patients. Dual antiplatelet agents and statins were used in about nine out of 10 patients in each of the three groups, beta-blockers in about seven out of 10, and renin-angiotensin blockers in about six out of 10, with no statistically significant differences among the three groups of patients.
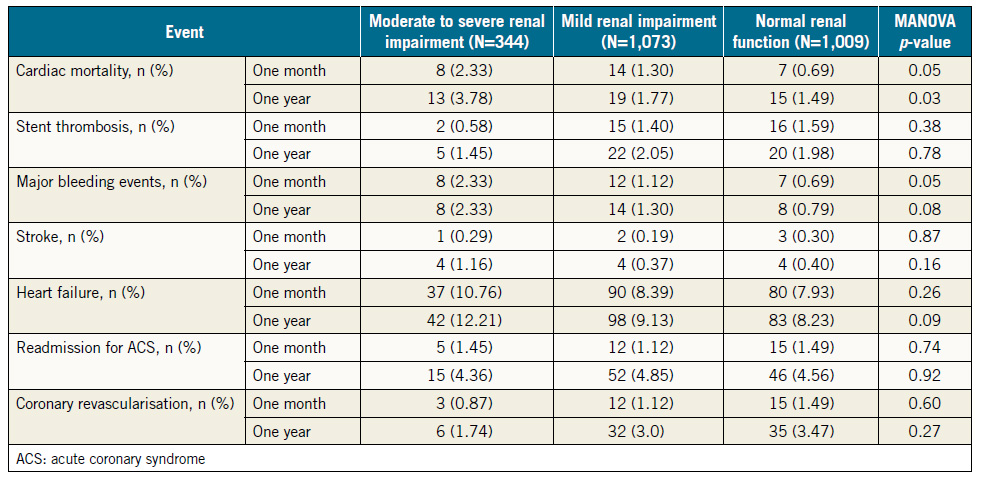
Cardiovascular events at one month and one year after the index hospitalisation are shown in Table 3. Patients with moderate to severe RI were at higher risk of cardiac death at one month and at one year.
As for the incidence of major bleeding events at one month and one year, patients with moderate to severe RI did not have excess events compared with patients with mild RI (2.3% vs. 1.1%, p=0.1 at one month, and 2.3% vs. 1.3%, p=0.2 at one year). However, these patients had significantly higher incidence of major bleeding compared with those with normal renal function at one month (2.3% vs. 0.7%, p=0.01), and at one year (2.3% vs. 0.8%, p=0.02).
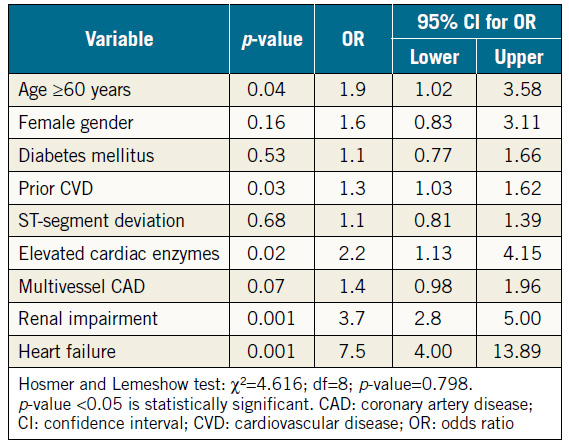
The impact of nine variables on cardiac mortality at one year was determined using the multivariate logistic regression model (Table 4). Renal impairment was a strong predictor of cardiac mortality at one year (odds ratio [OR] 3.7, 95% CI: 2.8-5.0, p=0.001). Other predictors included heart failure (OR 7.5, 95% CI: 4.0-13.9, p=0.001), elevated cardiac enzymes (OR 2.2, 95% CI: 1.1-4.2, p=0.02), age ≥60 years (OR 1.9, 95% CI: 1.0-3.6, p=0.04), and prior CVD (OR 1.3, 95% CI: 1.0-1.6, p=0.03).
Table 2. In-hospital complications and medications.
Discussion
The major findings of this study are that (1) in a contemporary cohort of Middle Eastern patients who underwent PCI, less than half (44%) had mild RI and 14% had moderate to severe RI, (2) patients with moderate to severe RI were older, more likely to be female and to have hypertension and DM compared with those with mild or no RI, (3) patients with moderate to severe RI had higher prevalence of multivessel CAD and more PCI for stable coronary disease than patients with mild or no RI, and (4) moderate to severe RI was an independent predictor of cardiac mortality. These findings are the first from this region to address specifically the impact of RI on cardiovascular outcomes, and will thus serve as assuring evidence for the practising cardiologists that PCI for patients with RI is both safe and feasible. The misconception that PCI is better avoided or delayed in this high-risk population is not supported by clinical trials. The current study will be the first to dissipate this misconception.
Impaired renal function, an important risk factor for CVD and a strong predictor of adverse events after ACS, has not been extensively studied in the Middle East despite the high prevalence of CVD and the increasing number of patients admitted with ACS3,4,10-13. Previous studies have indicated that, among ACS patients, 8 and 6 out of 10 undergo diagnostic coronary angiography or coronary intervention, respectively4, reflecting a high degree of adherence to guidelines that recommend an invasive strategy in these patients. The prevalence of moderate to severe RI reported in the current study (14%) is lower than the prevalence rates of 30-40% reported by investigators from other regions19,20, possibly due to the relatively younger age of the enrolled patients in this region.
Renal function in this study was assessed by the eGFR using the abbreviated MDRD15, which is the recommended method for estimation of the GFR in adult patients with CVD and is also a validated tool for the estimation of GFR in a typical office setting21. The online equation calculator is simpler and produces essentially similar results to the original equation22. Several other validated estimation equations for GFR that use easily obtained clinical data and laboratory test results are readily available, including the Cockcroft-Gault23 and Chronic Kidney Disease Epidemiology Collaboration24 formulas. These equations are more practical than the more accurate formal GFR measurement using iothalamate or a similar marker, but they are time-consuming, expensive, generally unsuitable for clinical practice, available in only a few centres, and certainly not cost-effective for kidney disease screening22. Regardless of the method used, it is advisable to estimate the GFR for patients admitted with ACS or those who are planned to undergo PCI to facilitate risk stratification according to the degree of RI25, and for identification of high-risk patients in whom close monitoring and vigilance could lower the incidence of adverse events.
Patients who are admitted with ACS and found to have RI are less frequently referred for invasive coronary diagnostic and revascularisation procedures, and less commonly treated with guidelinebased cardiovascular medications compared with patients who have normal renal function9. These patients also experience higher rates of adverse cardiovascular events regardless of the conservative or invasive therapeutic strategies adopted9,20,26. RI doubles the mortality rates in ACS patients compared with patients who have normal renal function27. Moreover, PCI in patients who have RI is associated with an increased risk of major adverse cardiovascular events during and after the procedure including death, major bleeding events, heart failure, cardiogenic shock and target vessel revascularisation28,29. Several pathophysiological factors that explain the excess cardiovascular adverse events in patients with RI include the coexistence of comorbidities and cardiovascular risk factors (old age, female gender, DM, and hypertension), elevated state of inflammatory and oxidative stress, high levels of matrix metalloproteinase that are implicated in destabilisation of the coronary plaque fibrous cap, heavy and complex CAD burden, and accelerated MI expansion30-32. Despite the high-risk clinical and angiographic profiles of patients who have RI and physicians’ reluctance to manage them aggressively, there is strong evidence of significant symptomatic benefits, reduction of short- and longterm adverse events, and improved prognosis when such patients are referred to an invasive strategy to treat ACS regardless of the degree of RI33.
The relatively younger age, high prevalence of single-vessel CAD in more than half of all the patients, enrolling only patients who underwent PCI, the high rate of utilisation of medications shown to be associated with improved outcome including dual antiplatelet agents, renin-angiotensin system antagonists and statins, and the almost exclusive use of drug-eluting stents in the current study might be potential explanations for the absence of excess non-fatal in-hospital events in patients with RI32-34. It is very reassuring that the incidence of significant complications during the index hospitalisation was generally low and less than 1% in patients with moderate to severe RI for each single complication, except for heart failure (9.9%) and major bleeding events (2.0%). Furthermore, this study has clearly demonstrated that, among patients with variable degrees of RI, PCI is generally safe and effective in the long run with a one-year event rate comparable to or better than those observed by other investigators from other regions. This should encourage cardiologists in this region to refer this high-risk group of ACS patients to an invasive strategy in this contemporary era of interventional practice.
Management of ACS in the vulnerable patient group with severe RI and end-stage kidney disease requiring haemodialysis is challenging due to the high cardiovascular morbidity and mortality even after surgical or percutaneous coronary revascularisation34. In this study, only a minority of patients (0.8% and 1.1%) had very severe RI or end-stage renal disease requiring haemodialysis, respectively; thus, their impact on the overall incidence of adverse events is limited.
In agreement with other studies, PCI patients in this study who had moderate to severe RI had a higher rate of major bleeding events from the index hospital admission to one year of followup32,35. The bleeding risk is related to the prolonged bleeding time, abnormal platelet aggregation and adhesion, and the exaggerated response to and use of inappropriate doses of antiplatelet and fibrinolytic agents32,35.
The in-hospital cardiac mortality rates observed in this study among the patients with RI (≤1%) were lower than those reported by other regional registries that ranged between 12% and 40% in STEMI patients and from 0.8% to 13% in ACS patients depending on the degree of RI10-12. Cardiac mortality at one year in this study was significantly higher in patients with moderate to severe RI compared with those who had mild or no RI, in concordance with the findings of other investigators34- 36. Older age, female gender and excess bleeding events could all contribute to the high cardiac mortality rate. However, after correction for other factors associated with mortality risk, moderate to severe RI was an independent predictor of one-year mortality.
Table 3. Adverse cardiovascular events one month and one year after hospital discharge.
Table 4. Multivariate logistic regression model for one-year cardiac mortality.
Limitations
The current study has a few limitations. Data were collected from an observational study and might be subject to potential bias. ACS was defined according to current guidelines. However, the method of case identification may not have been completely specific, because patients with RI are more likely to have elevated levels of cardiac markers. The GFR estimated at admission reflected the renal function before any intervention during the index hospitalisation. The renal function was not re-evaluated pre-discharge or during follow-up and thus worsening or improvement of renal function could not be ruled out. Larger studies are needed to define better the impact on renal dysfunction, especially in those with severe RI and/or on haemodialysis, in all ACS patients, whether they are treated conservatively or by an invasive strategy, and also to re-evaluate renal function during the study course.
Conclusions
In this Middle Eastern patient population who had PCI, the presence of moderate to severe RI was an independent predictor for in-hospital and one-year cardiac mortality and major bleeding events. Estimating the GFR in such a population is strongly recommended in order to define better high-risk groups among these patients such that vigilance can be exercised for adverse cardiovascular events.
Impact on daily practice
The relatively low incidence rate of adverse cardiovascular events in a PCI-exclusive group should encourage healthcare professionals to refer ACS patients with RI to an invasive strategy.
Funding
The study was supported by an unrestricted grant from AstraZeneca.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364: 937-52.
2. McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS; INTERHEART Investigators. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581-9.
3. Al Suwaidi J. Acute coronary syndrome in the Middle East. Curr Atheroscler Rep. 2011;13:287-9.
4. Hammoudeh A, Izraiq M, Ismail Y, Tabbalat R, El-Harasis A, Al-Tarawneh H, Hamdan H, Al-Mousa E. Utilization of reperfusion therapy in the Myocardial INfarction Triggers and Onset in JoRdan (MINTOR) study. Int J Cardiol. 2007;122: 156-7.
5. Yuan J, Zou XR, Han SP, Cheng H, Wang L, Wang JW, Zhang LX, Zhao MH, Wang XQ; C-STRIDE study group. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2017; 18:23.
6. Rott D, Klempfner R, Goldenberg I, Matetzky S, Elis A. Temporal trends in the outcomes of patients with acute myocardial infarction associated with renal dysfunction over the past decade. Eur J Intern Med. 2016;29:88-92.
7. Baber U, Farkouh ME, Arbel Y, Muntner P, Dangas G, Mack MJ, Hamza TH, Mehran R, Fuster V. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J. 2016;37: 3440-7.
8. Rozenbaum Z, Leader A, Neuman Y, Shlezinger M, Goldenberg E, Mosseri M, Pereg D. Prevalence and Significance of Unrecognized Renal Dysfunction in Patients with Acute Coronary Syndrome. Am J Med. 2016;129:187-94.
9. Zannad F, Rossignol P. Cardiovascular Outcome Trials in Patients With Advanced Kidney Disease: Time for Action. Circulation. 2017;135:1769-71.
10. Zubaid M, Rashed WA, Almahmeed W, Al-Lawati J, Sulaiman K, Al-Motarreb A, Amin H, Al Suwaidi J, Alhabib K. Management and outcomes of Middle Eastern patients admitted with acute coronary syndromes in the Gulf Registry of Acute Coronary Events (Gulf RACE). Acta Cardiol. 2009;64:439-46.
11. Alhabib KF, Sulaiman K, Al-Motarreb A, Almahmeed W, Asaad N, Amin H, Hersi A, Al-Saif S, AlNemer K, Al-Lawati J, Al-Sagheer NQ, AlBustani N, Al Suwaidi J; Gulf RACE-2 investigators. Baseline characteristics, management practices, and longterm outcomes of Middle Eastern patients in the second Gulf Registry of Acute Coronary Events (Gulf RACE-2). Ann Saudi Med. 2012;32:9-18.
12. El-Menyar A, Zubaid M, Sulaiman K, Singh R, Al Thani H, Akbar M, Bulbanat B, Al-Hamdan R, Almahmmed W, Al Suwaidi J. In-hospital major clinical outcomes in patients with chronic renal insufficiency presenting with acute coronary syndrome: data from a registry of 8176 patients. Mayo Clin Proc. 2010;85:332-40.
13. AlFaleh HF, Alsuwaida AO, Ullah A, Hersi A, AlHabib KF, AlShahrani A, AlNemer K, AlSaif S, Taraben A, Ahmed WA, Balghith MA, Kashour T. Glomerular filtration rate estimated by the CKD-EPI formula is a powerful predictor of in-hospital adverse clinical outcomes after an acute coronary syndrome. Angiology. 2011;63:119-26.
14. Alhaddad IA, Tabbalat R, Khader Y, Al-Mousa E, Izraiq M, Nammas A, Jarrah M, Saleh A, Hammoudeh A; First Jordanian PCI Registry Investigators Group. Outcomes of Middle Eastern Patients Undergoing Percutaneous Coronary Intervention: The Primary Analysis of the First Jordanian PCI Registry. Heart Views. 2017;18:3-7.
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130: 461-70.
16. Levin A, Stevens PE, Bilous RW, De Francisco AL, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Rielle MC, Shlipak MG, Wang H, White CT, Winearls CG. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
17. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L,Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115: 2344-51.
18. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non-ST-segment elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Bleeding Score. Circulation. 2009; 119:1873-82.
19. Bachorzewska-Gajewska H, Malyszko J, Malyszko JS, Dobrzycki S, Sobkowicz B, Musial W. Estimation of glomerular filtration rate in patients with normal serum creatinine undergoing primary PCI: is it really normal? Nephrol Dial Transplant. 2006;21:1736-8.
20. Goldenberg I, Subirana I, Boyko V, Vila J, Elosua R, Permanyer-Miralda G, Ferreira-Gonzalez I, Benderly M, Guetta V, Behar S, Marrugat J. Relation between renal function and outcomes in patients with non-ST segment elevation acute coronary syndrome: real-world data from the European Public Health Outcome Research and Indicators Collection Project. Arch Intern Med. 2010;170:888-95.
21. Brosius FC 3rd, Hostetter TH, Kelepouris E, Mitsnefes MM, Moe SM, Moore MA, Pennathur S, Smith GL, Wilson PW; American Heart Association Kidney and Cardiovascular Disease Council; Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; Quality of Care and Outcomes Research Interdisciplinary Working Group; National Kidney Foundation. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a science advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: Developed in Collaboration With the National Kidney Foundation. Hypertension. 2006; 48:751-5.
22. Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14: 2573-80.
23. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41.
24. Inal BB, Oguz O, Emre T, Usta M, Inal H, Altunoglu E, Topkaya C. Evaluation of MDRD, Cockcroft-Gault, and CKD-EPI formulas in the estimated glomerular filtration rate. Clin Lab. 2014; 60:1685-94.
25. Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, Southern DA, McLaughlin K, Mortis G, Culleton BF. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155-61.
26. Chan W, Ivanov J, Ko D, Fremes S, Rao V, Jolly S, Cantor WJ, Lavi S, Overgaard CB, Ruel M, Tu JV, Dzavík V. Clinical outcomes of treatment by percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with chronic kidney disease undergoing index revascularization in Ontario. Circ Cardiovasc Interv. 2015;8:e001973.
27. Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113-9. 28. Sica D. The implications of renal impairment among patients undergoing percutaneous coronary intervention. J Invasive Cardiol. 2002;14 Suppl B:30B-37B.
29. Drueke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723-35.
30. Naito K, Anzai T, Yoshikawa T, Anzai A, Kaneko H, Kohno T, Takahashi T, Kawamura A, Ogawa S. Impact of chronic kidney disease on postinfarction inflammation, oxidative stress, and left ventricular remodeling. J Card Fail. 2008;14:831-8.
31. Hu LH, Zhang LJ, Jin ZT, Yang W, Zhang LN, Lu CY. Analysis of the Clinical Characteristics of Patients with Acute Coronary Syndrome in Different States of Renal Function. West Indian Med J. 2015;64:357-61.
32. Ibrahim H, Rao SV. Oral antiplatelet drugs in patients with chronic kidney disease (CKD): a review. J Thromb Thrombolysis. 2017;43:519-27.
33. Möckel M, Searle J, Baberg HT, Dirschedl P, Levenson B, Malzahn J, Mansky T, Günster C, Jeschke E. Revascularization of patients with end-stage renal disease on chronic hemodialysis: bypass surgery versus PCI-analysis of routine statutory health insurance data. Open Heart. 2016;3:e000646.
34. Gutierrez A, Rao SV. Incidence, outcomes, and management of bleeding in non-ST-elevation acute coronary syndromes. Cleve Clin J Med. 2010;77:369-79.
35. Perdoncin E, Zhang M, Riba A, LaLonde TA, Grines CL, Gurm HS. Impact of worsening renal dysfunction on the comparative efficacy of bivalirudin and platelet glycoprotein IIb/ IIIa inhibitors: insights from Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Circ Cardiovasc Interv. 2013;6: 688-93. 36. Franczyk-Skora B, Gluba A, Banach M, Rozentryt P, Polonski L, Rysz J. Acute coronary syndromes in patients with chronic kidney disease. Curr Vasc Pharmacol. 2013;11:758-67.
To download, please click below.