Kohsuke Shirakawa1, MD, PhD; Yuji Itabashi1*, MD, PhD; Hikaru Tsuruta1, MD; Jin Endo1, MD, PhD; Yugo Minakata1, MD; Kentaro Hayashida1, MD, PhD; Takahide Arai1, MD, PhD; Ryo Yanagisawa1, MD; Makoto Tanaka1, MD; Hideyuki Shimizu2, MD, PhD; Keiichi Fukuda1, MD, PhD; Mitsushige Murata3, MD, PhD
AsiaIntervention 2019;5:72-80, DOI: 10.4244/AIJ-D-18-00021
1. Department of Cardiology, Keio University School of Medicine, Tokyo, Japan; 2. Department of Cardiovascular Surgery, Keio University School of Medicine, Tokyo, Japan; 3. Department of Laboratory Medicine, Keio University School of Medicine, Tokyo, Japan
Abstract
Aims: Increased stroke volume (SV) is a prognosticator of severe aortic stenosis (AS) after transcatheter aortic valve replacement (TAVR). This study aimed to investigate preprocedural echocardiographic predictors of increased SV after TAVR.
Methods and results: Clinical and echocardiographic data were retrospectively analysed in 129 patients with severe AS who underwent TAVR (2013-2015). We compared the echocardiographic data and cardiac events between the decreased SV group (n=28) and the increased SV group (n=101). Univariate and multivariate analyses were used to assess the predictors of increasing SV. AS severity significantly diminished, left and right ventricular function improved, and SV index (SVi) increased after TAVR: aortic valve area index (0.46±0.13 vs. 1.18±0.33 cm2, p<0.001); aortic regurgitation (AR) grade (1.85±0.55 vs. 1.60±0.54, p<0.001); left ventricular ejection fraction (59.9±12.7 vs. 64.1±12.0%, p<0.001); right ventricular fractional area change (RVFAC) (48.8±11.9 vs. 53.3±14.0%, p<0.001); SV index (SVi) (46.7±11.0 vs. 52.8±12.0 ml/m2, p<0.001). Kaplan-Meier survival estimates suggested that the SVi increase was associated with the decreased cardiovascular events one year after TAVR (hazard ratio 4.08, 95% confidence interval [CI]: 1.32-12.7, p=0.02). On multivariate analysis, preprocedural AR grade (odds ratio [OR] 7.00, 95% CI: 2.76-17.8, p<0.001) and preprocedural RVFAC (OR 1.05, 95% CI: 1.01-1.10, p=0.011) correlated with the SV increase.
Conclusions: Preprocedurally, greater AR and higher RVFAC could predict an increased SVi and thus the occurrence of fewer cardiac events. Preserved preprocedural RV systolic function is crucial for an increased SV after TAVR.
Abbreviations
3D: three-dimensional
3D-RVEF: three-dimensional right ventricular ejection fraction
AR: aortic regurgitation
AS: aortic stenosis
CI: confidence interval
LVEDV: left ventricular end-diastolic volume
LVEF: left ventricular ejection fraction
LVESV: left ventricular end-systolic volume
LVOT: left ventricular outflow tract
MR: mitral regurgitation
OR: odds ratio
PVL: paravalvular leak
RVEDA: right ventricular end-diastolic area
RVESA: right ventricular end-systolic area
RVFAC: right ventricular fractional area change
RV S′: right ventricular S′
S’: pulsed Doppler peak velocity at the annulus
SV: stroke volume
TAVR: transcatheter aortic valve replacement
TR: tricuspid regurgitation
TTE: transthoracic echocardiography
Introduction
Cardiac output is strongly related to the symptoms and prognosis of patients with severe aortic stenosis (AS)1. Recently, several studies have shown the impact of stroke volume (SV) on the prognosis of AS patients following transcatheter aortic valve replacement (TAVR)2,3. Herrmann et al demonstrated that preprocedural low flow independently predicted mortality and was a more powerful predictor of outcome than left ventricular ejection fraction (LVEF) or the mean transaortic pressure gradient of patients following TAVR4. Anjan et al demonstrated that severe low flow at discharge was associated with an increased risk of mortality2, and Le Ven et al showed that increased SV following TAVR resulted in better long-term outcomes among patients with preprocedural low-flow AS3. These studies suggest that increased SV is a prognostic predictor in patients following TAVR.
There are several possible mechanisms for increased SV following TAVR, including improved left and right heart function and less severe valvular regurgitation. However, there have been few investigations of the haemodynamic parameters related to increased SV following TAVR. We therefore investigated predictors of increased SV following TAVR, evaluating the preprocedural echocardiographic parameters.
Methods
STUDY POPULATION
We retrospectively reviewed 140 consecutive patients who underwent TAVR from October 2013 to September 2015 in our institution. Among those patients, three did not have follow-up transthoracic echocardiography (TTE) records because they died during the acute phase after TAVR, and there was insufficient image quality for assessing right ventricular fractional area change (RVFAC) in eight, leaving 129 patients who were evaluated. The one-year (336.8±6.8 days) assessment after TAVR revealed that heart failure, ischaemic heart disease, arrhythmia requiring admission, and cardiac death comprised the cardiac events that had occurred during this period. Patient selection for TAVR conformed to a standard process that comprised clinical evaluation, multidetector computed tomography scanning, and echocardiography prior to when decisions about treatment were made by our multidisciplinary Heart Team. All patients received an Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA). Data were retrieved from our computerised database, and clinical information was obtained retrospectively for all patients. The institutional review board of our institution approved the study.
COMPREHENSIVE TRANSTHORACIC ECHOCARDIOGRAPHY
Comprehensive TTE studies were performed at baseline (42.2±2.8 days before TAVR) and after TAVR (2.1±0.2 days). All TTE studies were obtained using a Philips iE33 ultrasonography system (Philips Medical Systems, Best, the Netherlands) and Vivid E9 or Vivid 7 ultrasonography system (GE Healthcare, Chicago, IL, USA). They were evaluated according to the guidelines of the American Society of Echocardiography5.
Left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were measured using the method of discs (modified Simpson’s rule), and the LVEF was calculated. The stroke volume (SV) was calculated according to the guideline using pulsed-wave Doppler recording at the left ventricular outflow tract (LVOT)5. The aortic valve area was calculated using the continuity equation. The area of the right ventricle was measured by outlining the endocardial borders at end-diastole and end-systole in the apical four-chamber view. The RVFAC was calculated as follows:
[RVEDA – RVESA]/RVEDA
where RVEDA is the right ventricular end-diastolic area, and RVESA is the right ventricular end-systolic area. Mitral regurgitation (MR) severity was graded as none-to-mild, moderate, moderate- to-severe, or severe, based primarily on the jet area and vena contracta width of the MR jet5. Preprocedural aortic regurgitation (AR) severity was graded according to the same guideline based primarily on the jet width and vena contracta5. The post-procedural AR severity was graded according to the American Society of Echocardiography guideline6 or other recommendations7, based primarily on the circumferential extent of paravalvular regurgitation (PVL) and jet area evaluated on multiple TTE planes.
DEFINITION OF INCREASED/DECREASED SV AND CARDIOVASCULAR EVENTS ONE YEAR AFTER TAVR
The increase in SV was defined as the post-procedural SV/preprocedural SV >1. Otherwise, the SV was considered to have worsened. Cardiovascular events were assessed at the one-year follow-up visit following TAVR. Primary cardiovascular events were heart failure, ischaemic heart disease, or arrhythmia requiring admission, and cardiac death.
STATISTICAL ANALYSIS
Data are presented as numbers with percentages for categorical variables or as means±standard deviations (SD) for continuous variables. Categorical variables were compared with a χ2 test or Fisher’s exact test, as appropriate. Differences between groups were analysed by a paired or unpaired t-test in case of normal distribution or by the Wilcoxon or the Mann-Whitney U test, as appropriate, in case of non-normal distribution. Cumulative event rates were calculated using the Kaplan-Meier estimates for the increases and decreases in SV after TAVR. The log-rank test for time-to-event data at one year for cardiac events was used for statistical comparison. Multiple logistic regressions were used to identify factors associated with increased SV after TAVR. Variables with probability values <0.20 in individual analyses were included in the multivariate analysis. We analysed intraobserver and inter-observer reproducibility for preprocedural and post-procedural LVEF, RVFAC, and SV from TTE data in 15 randomly selected patients and expressed them using the Bland- Altman analysis. A two-sided value of p<0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS, Version 21.0 software (IBM Corp., Armonk, NY, USA).
Results
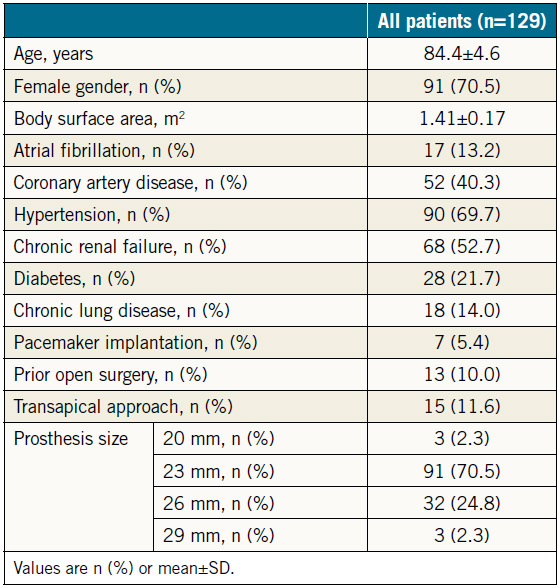
BASELINE CHARACTERISTICS AND PREPROCEDURAL AND POST-PROCEDURAL ECHOCARDIOGRAPHIC MEASUREMENTS
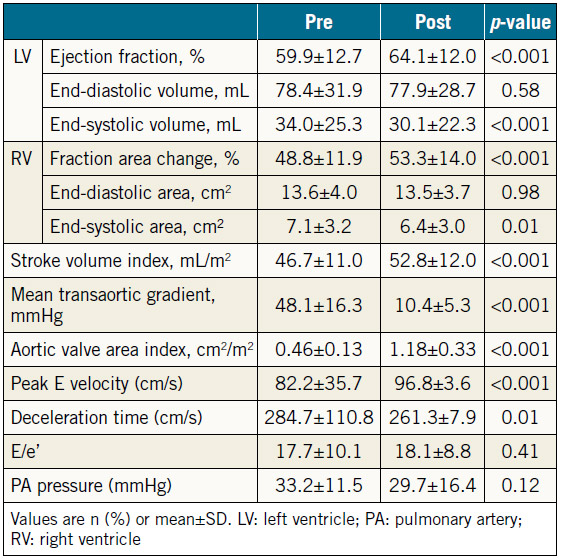
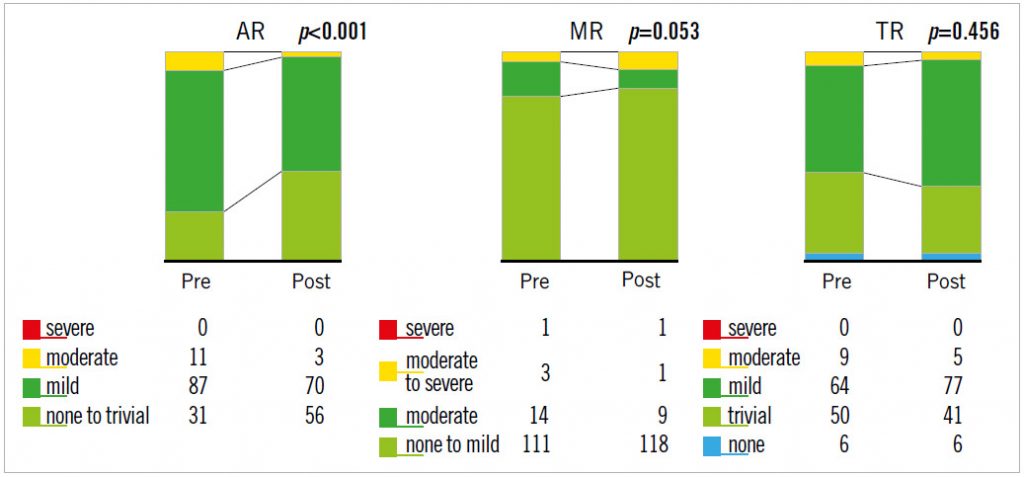
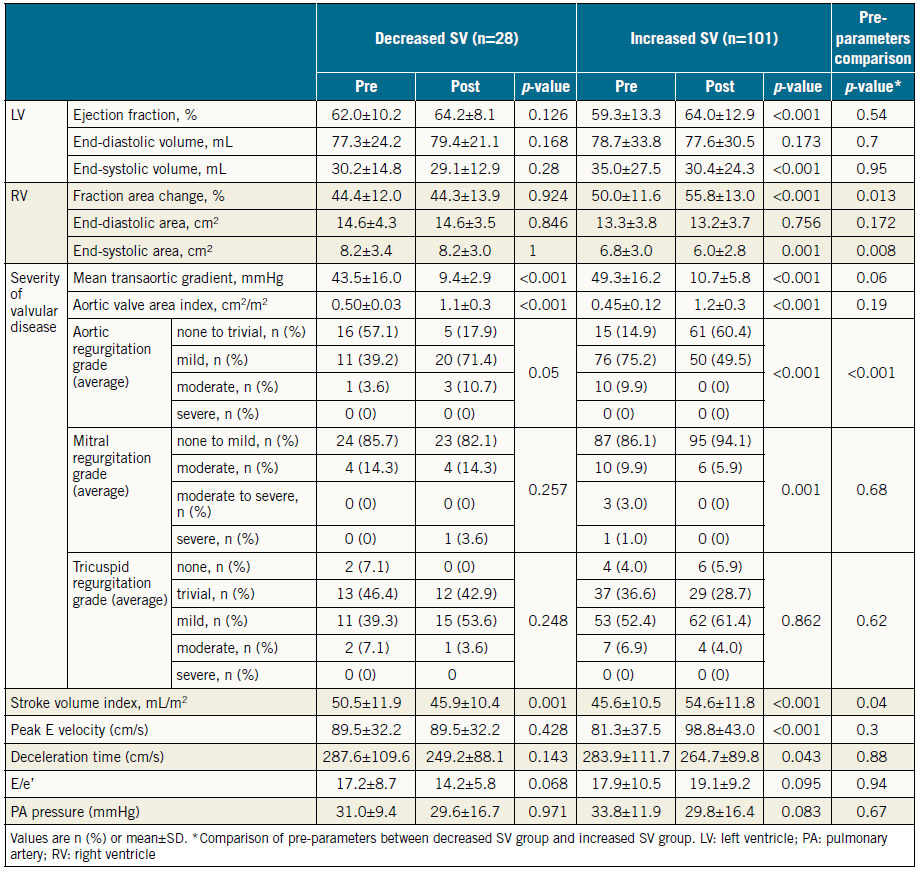
The baseline data for the 129 patients are shown in Table 1. SAPIEN XT valve diameters during the study period were 20, 23, 26, and 29 mm. The echocardiographic changes are shown in Table 2. Despite no significant difference in the LVEDV and RVEDA, the LVESV and RVESA decreased following TAVR. As a result, the LVEF and RVFAC had improved postoperatively. AS severity, assessed by mean transaortic pressure gradient and aortic valve area index, diminished following TAVR. The AR grade improved in 46 patients (35.6%), did not change in 67 patients (51.9%), and worsened in 16 patients (12.4%). There were also three patients whose AR severity was moderate or greater after TAVR (2.3%). The MR and tricuspid regurgitation (TR) grades did not change significantly following TAVR (Figure 1). Stroke volume index (SVi) increased significantly after TAVR.
Table 1. Baseline and procedural characteristics.
Table 2. Preprocedural and post-procedural echocardiographic parameters of all patients undergoing TAVR (n=129).
BASELINE CHARACTERISTICS AND CARDIAC EVENTS OF INCREASED OR DECREASED SV PATIENTS AFTER TAVR
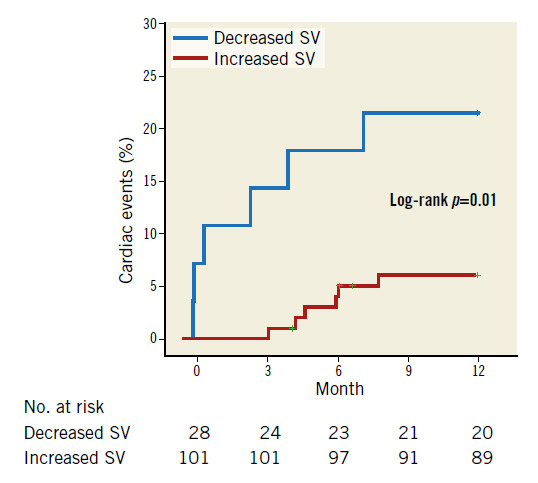
Overall, 12 patients had cardiac events (one cardiac death and 10 admissions for heart failure, one for sick sinus syndrome) during the observational period (336.8±6.8 days). There was no significant difference in the history of coronary artery disease (42.9% vs. 39.6%, p=0.76) or in the percentage of the transapical approach (10.7% vs. 11.9%, p=0.58) between patients with increased or decreased SV after TAVR. Kaplan-Meier survival estimates suggested that increased SV was associated with fewer cardiovascular events one year after TAVR (hazard ratio [HR] 4.08, 95% CI: 1.32-12.7, p=0.02) (Figure 2).
PREPROCEDURAL AND POST-PROCEDURAL ECHOCARDIOGRAPHIC MEASUREMENTS
Table 3 shows preprocedural and post-procedural echocardiographic measurements. Patients with increased SV had greater RVFAC and AR severity than those with decreased SV before TAVR. There was no significant difference in LVEF or the severity of MR or TR. The mean transaortic gradient decreased significantly after TAVR in both groups, whereas increased LVEF and RVFAC and less severe AR and MR were observed in patients with increased SV.
PREDICTORS OF IMPROVED SV AFTER TAVR
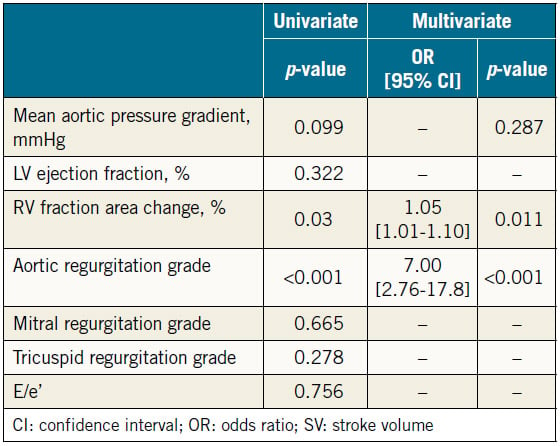
Results of univariate and multivariate analyses of preprocedural parameters of the severity of valvular diseases and LV or RV function on increased SV are shown in Table 4. In the univariate analysis, we selected a value of p<0.2 to indicate significance for the mean aortic pressure gradient (OR 1.02, 95% CI: 1.00- 1.05, p=0.099), RVFAC (OR 1.04, 95% CI: 1.00-1.08, p=0.030), and AR grade (OR 5.868, 95% CI: 2.46-14.0, p<0.001). In the multivariate analysis, RVFAC (OR 1.05, 95% CI: 1.01-1.10, p=0.011) and AR grade (OR 7.00, 95% CI: 2.76-17.8, p<0.001) were found to be independent predictors of increased SV after TAVR.
INTRA-OBSERVER AND INTER-OBSERVER VARIABILITY
The intra-observer variability for the TTE measurements, which was demonstrated by the 95% CI of the Bland-Altman method, was as follows: preprocedural LVEF −2.6 to 1.9%, RVFAC −3.4 to 4.3%, SV −4.8 to 3.8 mL, and post-procedural LVEF −2.7 to 1.4%, RVFAC −2.8 to 1.7%, SV −5.6 to 4.3 mL. The interobserver variability differences were as follows: preprocedural LVEF −2.8 to 1.9%, RVFAC −5.0 to 5.0%, SV −2.5 to 3.9 mL, and post-procedural LVEF −3.0 to 2.2%, RVFAC −2.5 to 3.4%, SV −3.0 to 3.6 mL.
Figure 1. Changes in valvular regurgitation following TAVR.
Figure 2. Kaplan-Meier plots for cardiac events between decreased and increased SV. Increased SV was associated with decreased cardiovascular events one year after TAVI (HR 4.08; 95% CI: 1.32-12.7, p=0.02).
Discussion
We showed that the increased SV afforded by TAVR led to a significant decrease in cardiac events, including cardiac death and readmission due to heart failure within one year. TAVR influenced haemodynamic parameters as follows: 1) the LVEF and RVFAC increased after TAVR due to the decrease in LVESV and the systolic RV area, and 2) the severity of AR, but not of MR, was significantly diminished following TAVR. In addition, the greater severity of AR and higher RVFAC before TAVR were correlated with the increased SV following TAVR.
Table 3. Preprocedural and post-procedural echocardiographic parameters of patients with decreased SV or increased SV following TAVR.
Table 4. Univariate and multivariate logistic regression analyses for estimating increasing SV (n=129).
CLINICAL IMPLICATION OF THE INCREASED SV
As Anjan et al previously reported, not only preprocedural but also post-procedural low flow were predictors of a poor prognosis following TAVR2. Le Ven et al showed that six-month and one-year all-cause mortality of patients with normalised flow following TAVR was lower than that in those with persistent low flow regardless of their preprocedural SV3. These findings suggested that increased SV was the important beneficial effect of TAVR. SV tends to be calculated higher by the Doppler method at the LVOT compared with the Simpson method in the previous validation study8; our data were consistent with that report. We adopted SV calculated by the Doppler method as previously reported and demonstrated that patients with decreased SV had a higher risk of cardiac events than those with increased SV at the one-year follow-up after TAVR2,3. These results are consistent with previous studies (Figure 2).
INFLUENCE OF THE SEVERITY OF VALVULAR DISEASE ON SV
Several studies have reported that paravalvular leak (PVL) has a negative impact on midterm and long-term prognosis following TAVR9,10. Consequently, preventing PVL is an important requirement for TAVR. We showed herein that the severity of AR was significantly reduced following TAVR in patients with increased SV but not in those with decreased SV (Table 3). Furthermore, the improved AR grade correlated with the increase in SV in all patients. The multivariate analysis showed that more severe AR before TAVR was an independent predictor of the subsequent increase in SV, which is consistent with the results of a previous study2. Because the severity of post-TAVR AR was rated almost always “mild or less” (126/129) in our study, patients with significant AR prior to TAVR could have had a greater reduction of AR volume following TAVR, resulting in the significantly increased SV. These results suggest that the reduced AR volume, even if it was from mild to trivial, is an important therapeutic effect of TAVR in addition to expansion of a narrowed aortic valve area. Thus, sophisticated techniques designed to leave no PVL are required. We need more data to clarify the mechanism by which the reduced AR improved the SV index after TAVR.
Previous studies have reported that preprocedural significant MR predicted a poor prognosis after TAVR, and that following TAVR 38.0-77.7% of patients exhibited significantly diminished MR severity11-14. In the present study, however, the severity of MR did not change significantly and was not correlated with the improved SV after TAVR in any patients (Figure 1, Table 4). These results indicate that the improved AR may have greater impact on the increase in SV than MR following TAVR.
INFLUENCE OF LEFT AND RIGHT VENTRICULAR FUNCTION ON SV
Several studies, including the PARTNER trial (Placement of Aortic Transcatheter Valves), showed that a preprocedural low ejection fraction was an independent predictor of a poor prognosis9,15,16, whereas others reported that it was not17-19. Thus, the results of previous studies are controversial. In our study, the reduction in LVESV led to increased LVEF after TAVR, and increased LVEF was observed in patients with increased SV. Preprocedural LVEF, however, was not an independent predictor of the increased SV following TAVR. The LVEF reflects the contractility of the left ventricle. It does not, however, accurately reflect the forward flow itself because of the influence of other haemodynamic factors. For instance, because aortic or mitral regurgitation (which fluctuates dynamically following TAVR) interferes with LVEF, preprocedural LVEF might not have independently predicted the increase in SV in the present study.
Several reports on RV function in patients following TAVR have described using parameters such as tricuspid annular plane systolic excursion, three-dimensional right ventricular (3D-RV) volume, RVFAC, right ventricular S′ (RV S′), TR severity, and pulmonary arterial systolic pressure. The effect of RV function on prognosis after TAVR, however, is not widely recognised20,21. The 3D right ventricular ejection fraction (3D-RVEF), measured by 3D transoesophageal echocardiography (3D-TEE) or magnetic resonance imaging, is a reliable parameter with respect to RV systolic function22-24. Lindsay et al reported that low 3D-RVEF was a predictor of poor prognosis in patients undergoing TAVR, but LVEF was not21. We adopted RVFAC – a conventional, accurate parameter of RV contraction in patients undergoing TAVR – to evaluate RV function25,26. RVFAC was significantly increased after TAVR in patients with increased SV but not in those with decreased SV. In addition, preprocedural RVFAC was significantly higher in patients with increased SV than in those with decreased SV, and the multivariate analysis showed that preprocedural RVFAC was an independent predictor of increased SV following TAVR. There were no significant differences in the other parameters that influence RVFAC, such as preprocedural LVEF, TR severity, and morbidity associated with chronic obstructive pulmonary disease between the two groups. Actually, preprocedural RVFAC independently predicted increasing SV; however, the odds ratio was low. The reason may be that RVFAC reflected forward flow less accurately compared with 3D-RVEF. Because RV function is composed of various factors – not only RVFAC but also RV strain, 3D-RVEF, RV S′, and RV myocardial performance index (MPI) – we need more analyses of these parameters in the future. Our results suggest that preserved preprocedural RV systolic function is important in TAVR patients because it affects their SV after TAVR.
Limitations
The study has limitations. The analyses were retrospective and observational. We were unable to measure all RV parameters, including the tricuspid annular plane systolic excursion or RV S′ in every patient. Of note, 3D-RVEF may be a more accurate parameter than RVFAC. In the present retrospective study, however, we could not accumulate an adequate number of cases with 3D-RVEF data. Therefore, we adopted RVFAC as the indicator of RV systolic function based on previous studies24. Because RV function is affected by LV function in general11, LV dysfunction might have resulted in low RVFAC in our study. Therefore, additional analyses focused on the LV-RV interaction in patients undergoing TAVR are now ongoing. Moreover, although the results of 28 patients with decreased SV suggested that the decreased preprocedural RVFAC was a predictor of decreased SV after TAVR, this finding should be investigated further in a larger group of patients.
Conclusions
Increased SV is an important therapeutic effect of TAVR associated with fewer cardiovascular events. Preprocedural higher AR grade and RVFAC could predict an increased SV. According to these results, the reduced AR volume by sophisticated techniques designed to leave no PVL and preserved preprocedural RV systolic function are crucial for increased SV after TAVR.
Impact on daily practice
An increased stroke volume index improves the outcomes of aortic stenosis patients following transcatheter aortic valve replacement (TAVR), as shown in previous studies. The present study revealed that preprocedural aortic regurgitation and right ventricular function are correlated with the increase in stroke volume after TAVR.
Acknowledgements
We thank Nancy Schatken, BS, MT(ASCP), from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
Y. Itabashi has received a grant from JSPS KAKENHI (2016-2020: 16K09452).
Conflict of interest statement
K. Shimizu and K. Hayashida are clinical proctors for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
References
1. Park SJ, Enriquez-Sarano M, Chang SA, Choi JO, Lee SC, Park SW, Kim DK, Jeon ES, Oh JK. Hemodynamic patterns for symptomatic presentations of severe aortic stenosis. JACC Cardiovasc Imaging. 2013;6:137-46.
2. Anjan VY, Herrmann HC, Pibarot P, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani VH, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Evaluation of Flow After Transcatheter Aortic Valve Replacement in Patients With Low-Flow Aortic Stenosis: A Secondary Analysis of the PARTNER Randomized Clinical Trial. JAMA Cardiol. 2016;1:584-92.
3. Le Ven F, Thébault C, Dahou A, Ribeiro HB, Capoulade R, Mahjoub H, Urena M, Nombela-Franco L, Allende Carrera R, Clavel MA, Dumont É, Dumesnil J, De Larochellière R, Rodés- Cabau J, Pibarot P. Evolution and prognostic impact of low flow after transcatheter aortic valve replacement. Heart. 2015;101: 1196-203.
4. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. 2013;127:2316-26.
5. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303-71.
6. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA Jr, Nakatani S, Quiñones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M; American Society of Echocardiography’s Guidelines and Standards Committee; Task Force on Prosthetic Valves; American College of Cardiology Cardiovascular Imaging Committee; Cardiac Imaging Committee of the American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography; American College of Cardiology Foundation; American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975-1014.
7. Pibarot P, Hahn RT, Weissman NJ, Monaghan MJ. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8:340-60.
8. Shahgaldi K, Manouras A, Brodin LA, Winter R. Direct measurement of left ventricular outflow tract area using three-dimensional echocardiography in biplane mode improves accuracy of stroke volume assessment. Echocardiography. 2010;27:1078-85.
9. Urena M, Webb JG, Eltchaninoff H, Munoz-Garcia AJ, Bouleti C, Tamburino C, Nombela-Franco L, Nietlispach F, Moris C, Ruel M, Dager AE, Serra V, Cheema AN, Amat-Santos IJ, de Brito FS, Lemos PA, Abizaid A, Sarmento-Leite R, Ribeiro HB, Dumont E, Barbanti M, Durand E, Alonso Briales JH, Himbert D, Vahanian A, Immè S, Garcia E, Maisano F, del Valle R, Benitez LM, García del Blanco B, Gutiérrez H, Perin MA, Siqueira D, Bernardi G, Philippon F, Rodés-Cabau J. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol. 2015;65:437-48.
10. Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning JM, Nickenig G, Capodanno D, Tamburino C, Latib A, Colombo A, Kapadia SR. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585-95.
11. Barbanti M, Webb JG, Hahn RT, Feldman T, Boone RH, Smith CR, Kodali S, Zajarias A, Thompson CR, Green P, Babaliaros V, Makkar RR, Szeto WY, Douglas PS, McAndrew T, Hueter I, Miller DC, Leon MB; Placement of Aortic Transcatheter Valve Trial Investigators. Impact of preoperative moderate/severe mitral regurgitation on 2-year outcome after transcatheter and surgical aortic valve replacement: insight from the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation. 2013;128:2776-84.
12. Cortes C, Amat-Santos IJ, Nombela-Franco L, Munoz- Garcia AJ, Gutierrez-Ibanes E, De La Torre Hernandez JM, Cordoba-Soriano JG, Jimenez-Quevedo P, Hernandez-Garcia JM, Gonzalez-Mansilla A, Ruano J, Jimenez-Mazuecos J, Castrodeza J, Tobar J, Islas F, Revilla A, Puri R, Puerto A, Gómez I, Rodés- Cabau J, San Román JA. Mitral Regurgitation After Transcatheter Aortic Valve Replacement: Prognosis, Imaging Predictors, and Potential Management. JACC Cardiovasc Interv. 2016;9:1603-14.
13. Kiramijyan S, Koifman E, Asch FM, Magalhaes MA, Didier R, Escarcega RO, Negi SI, Baker NC, Jerusalem ZD, Gai J, Torguson R, Okubagzi P, Wang Z, Shults CC, Ben-Dor I, Corso PJ, Satler LF, Pichard AD, Waksman R. Impact of Functional Versus Organic Baseline Mitral Regurgitation on Short- and Long-Term Outcomes After Transcatheter Aortic Valve Replacement. Am J Cardiol. 2016;117: 839-46.
14. Toggweiler S, Boone RH, Rodés-Cabau J, Humphries KH, Lee M, Nombela-Franco L, Bagur R, Willson AB, Binder RK, Gurvitch R, Grewal J, Moss R, Munt B, Thompson CR, Freeman M, Ye J, Cheung A, Dumont E, Wood DA, Webb JG. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59:2068-74.
15. Eleid MF, Goel K, Murad MH, Erwin PJ, Suri RM, Greason KL, Nishimura RA, Rihal CS, Holmes DR Jr. Meta- Analysis of the Prognostic Impact of Stroke Volume, Gradient, and Ejection Fraction After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2015;116:989-94.
16. Schaefer U, Zahn R, Abdel-Wahab M, Gerckens U, Linke A, Schneider S, Eggebrecht H, Sievert H, Figulla HR, Senges J, Kuck KH; German TAVI Registry. Comparison of outcomes of patients with left ventricular ejection fractions ≤30% versus ≥30% having transcatheter aortic valve implantation (from the German Transcatheter Aortic Valve Interventions Registry). Am J Cardiol. 2015;115:656-63.
17. Malkin CJ, Long WR, Baxter PD, Gale CP, Wendler O, Monaghan M, Thomas MT, Ludman PF, de Belder MA, Cunningham AD, Moat NE, Blackman DJ; National Institute for Cardiovascular Outcomes Research (NICOR). Impact of left ventricular function and transaortic gradient on outcomes from transcatheter aortic valve implantation: data from the UK TAVI Registry. EuroIntervention. 2016;11:1161-9.
18. Passeri JJ, Elmariah S, Xu K, Inglessis I, Baker JN, Alu M, Kodali S, Leon MB, Svensson LG, Pibarot P, Fearon WF, Kirtane AJ, Vlahakes GJ, Palacios IF, Douglas PS; PARTNER Investigators. Transcatheter aortic valve replacement and standard therapy in inoperable patients with aortic stenosis and low EF. Heart. 2015;101:463-71.
19. D’Onofrio A, Besola L, Rizzoli G, Bizzotto E, Manzan E, Tessari C, Bianco R, Tarantini G, Badano LP, Napodano M, Fraccaro C, Pittarello D, Gerosa G. Impact of Changes in Left Ventricular Ejection Fraction on Survival After Transapical Aortic Valve Implantation. Ann Thorac Surg. 2017;103:559-66.
20. Lindman BR, Maniar HS, Jaber WA, Lerakis S, Mack MJ, Suri RM, Thourani VH, Babaliaros V, Kereiakes DJ, Whisenant B, Miller DC, Tuzcu EM, Svensson LG, Xu K, Doshi D, Leon MB, Zajarias A. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv. 2015 Apr;8(4).
21. Lindsay AC, Harron K, Jabbour RJ, Kanyal R, Snow TM, Sawhney P, Alpendurada F, Roughton M, Pennell DJ, Duncan A, Di Mario C, Davies SW, Mohiaddin RH, Moat NE. Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv. 2016 Jul;9(7).
22. Crouch G, Bennetts J, Sinhal A, Tully PJ, Leong DP, Bradbrook C, Penhall AL, De Pasquale CG, Chakrabarty A, Baker RA, Selvanayagam JB. Early effects of transcatheter aortic valve implantation and aortic valve replacement on myocardial function and aortic valve hemodynamics: insights from cardiovascular magnetic resonance imaging. J Thorac Cardiovasc Surg. 2015;149:462-70.
23. Keyl C, Schneider J, Beyersdorf F, Ruile P, Siepe M, Pioch K, Schneider R, Jander N. Right ventricular function after aortic valve replacement: a pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur J Cardiothorac Surg. 2016;49:966-71.
24. Musa TA, Uddin A, Fairbairn TA, Dobson LE, Steadman CD, Kidambi A, Ripley DP, Swoboda PP, McDiarmid AK, Erhayiem B, Garg P, Blackman DJ, Plein S, McCann GP, Greenwood JP. Right ventricular function following surgical aortic valve replacement and transcatheter aortic valve implantation: A cardiovascular MR study. Int J Cardiol. 2016;223:639-44.
25. Demirkol S, Ozturk C, Balta S, Unlu M. Right ventricular function in patients undergoing surgical or transcatheter aortic valve replacement. Eur J Cardiothorac Surg. 2016;49:1296.
26. Okada DR, Rahmouni HW, Herrmann HC, Bavaria JE, Forfia PR, Han Y. Assessment of right ventricular function by transthoracic echocardiography following aortic valve replacement. Echocardiography. 2014;31:552-7.
To download, please click below.