Introduction
Coronary angiography is a conventional imaging technique used for the assessment of coronary arteries and the guidance of percutaneous coronary interventions (PCI)1. Intravascular imaging by means of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) provides additional information which may be used to enhance stent implantation and reduce stent-related problems23. Pre-PCI, OCT can help to evaluate plaque morphology, stent size and reference segments. During lesion preparation and stent deployment, OCT has been beneficial in minimising geographic miss and allows automated evaluation of stent expansion4. Post-PCI, OCT helps to determine apposition and identify edge dissection and tissue protrusion4. Despite improved clinical outcomes with intravascular imaging techniques, their adoption is limited in medical practice due to cost, the need for additional time, perception of complexity, and lack of training and expertise15.
According to the recent American College of Cardiology (ACC)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) Guidelines for coronary artery revascularisation, OCT is recommended as an alternative to IVUS for procedural guidance in patients undergoing coronary stent implantation6. The consensus document of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) recommends the adjunctive use of intravascular imaging for diagnostic evaluation of coronary artery disease and optimisation of PCI17. This consensus document by the EAPCI on the use of intravascular imaging has been endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter ENdo-cardiovascular Therapeutics (HKSTENT), and the Cardiac Society of Australia and New Zealand7. The European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on myocardial revascularisation recommend the use of OCT for the guidance of PCI8. The expert consensus document of the Japanese Association of Cardiovascular Intervention and Therapeutics also recommends the use of OCT-guided PCI910. The Asia Pacific consensus document on coronary bifurcation recommends the use of intravascular imaging in left main and non-left main stem bifurcation interventions11. However, similar local guidelines related to the use of OCT-guided PCI seem to be lacking in Southeast Asia (SEA). This may be one of the contributing factors for the low adoption of intravascular imaging in SEA.
The main objectives of this manuscript are (i) to understand the current clinical utility of imaging and OCT in SEA, (ii) to assess the barriers and enablers of imaging and OCT adoption across SEA, and (iii) to map a path for the future of intravascular imaging in SEA.
This article is based on the outcomes of the advisory panel meeting that was conducted in September 2021, among leading SEA interventional cardiologists, to discuss the recent updates and future developments related to intravascular imaging, OCT, and PCI optimisation, and to obtain insights into the current and future roles of these techniques in SEA.
Data collection
A pre-meeting survey was conducted to obtain the opinions of 10 regional experts (Brunei [n=1], Indonesia [n=1], Malaysia [n=3], Singapore [n=2], and Thailand [n=3]) from SEA related to (i) the current uptake and clinical utility of imaging and OCT in SEA, (ii) the barriers of imaging and OCT adoption across SEA, and (iii) the future of intravascular imaging in SEA. Survey questions are presented in Supplementary Table 1. Data from the questionnaire are summarised as counts and displayed as histograms.
Current uptake of imaging and OCT in SEA
According to the survey, the average imaging use among the experts was 51% while the maximum rate was 95%. The average rate of IVUS (54%) performed was higher compared with OCT (46%). On average, 9 OCT-guided procedures per month were performed by these experts in SEA. All of the experts from Brunei (n=1), Singapore (n=2) and Thailand (n=3) had been using OCT for more than 5 years. Most of the experts (2 of 3) from Malaysia and one expert from Indonesia had been using OCT for the past 2-5 years. One expert from Malaysia had used OCT for less than 2 years. A majority of the experts (90%) were highly interested in OCT. Nearly 70% of the experts stated that they may perform OCT in more than 80% of cases in the next 5 years. Representative images of common plaque morphology during OCT evaluation are demonstrated in Figure 1.
All experts stated that, at present, there are no local guidelines that influence their decision to use imaging/OCT in clinical practice in SEA. OCT was most commonly used for pre- and post-PCI assessment, as well as to guide and influence procedural strategy, to guide bifurcation, and to assess intermediate left main coronary artery lesions (Figure 2).
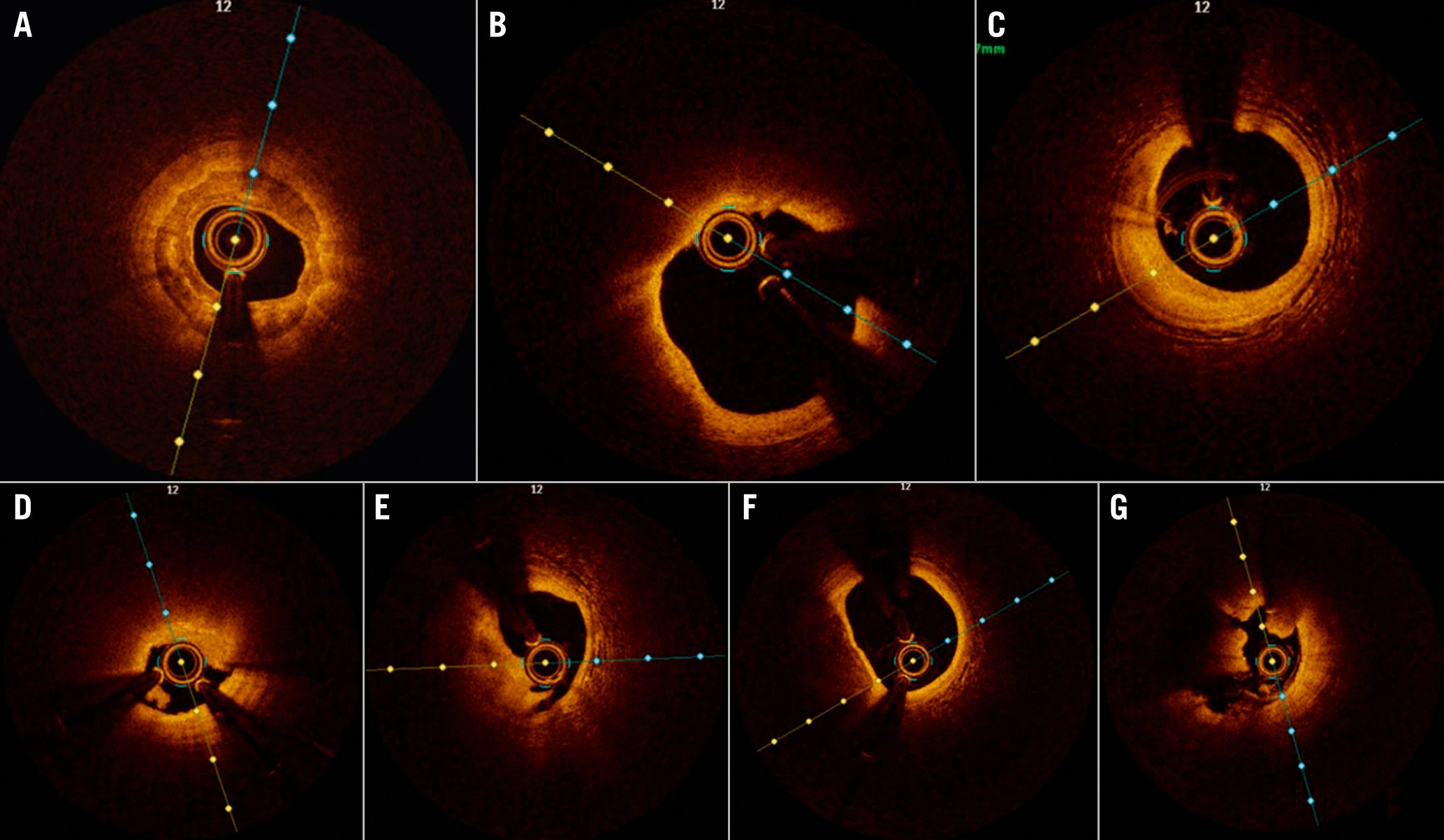
Figure 1. OCT images obtained during plaque assessment. A) Calcified plaque and calcium thickness; B) lipid plaque; C) fibrous plaque; D) plaque erosion; E) plaque rupture; F) thin cap fibroatheroma; G) red thrombus.
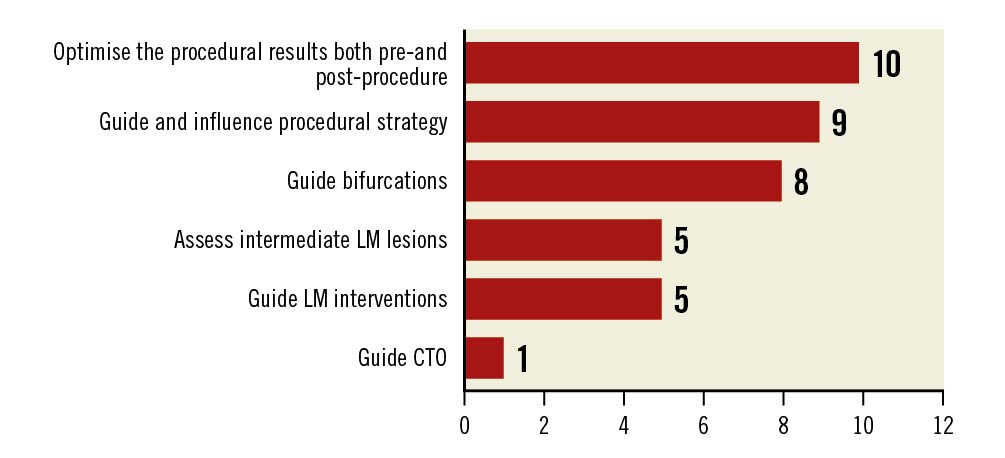
Figure 2. Clinical scenarios suitable for OCT. CTO: chronic total occlusions; LM: left main coronary artery; OCT: optical coherence tomography
KEY LEARNINGS
- OCT was preferred for certain lesion subsets, such as in cases of acute coronary syndrome (ACS; rupture vs erosion), calcified lesions, stent failure, and left main lesions (LML) in some instances, and bifurcations in a few cases.
- IVUS use was often preferred in LML, chronic total occlusion, and chronic kidney disease.
- OCT offered both better resolution compared to IVUS, along with easier interpretation.
- Current advances in OCT software have increased the ease of usage which aids quicker decision-making. This also ensures a systematic approach that improves the case outcomes.
- There is an interest among junior practitioners and fellows to use OCT under the supervision of experts.
Barriers to imaging and OCT adoption across SEA
The average estimated usage of imaging was low across the region (Malaysia 13%, Indonesia 16%, Singapore 20%, and Thailand 12%), with the exception of Brunei (80%). According to the survey, some common reasons for suboptimal use of imaging were the cost of the procedure, reimbursement, time needed to perform the test, and lack of familiarity with the technique (Figure 3). Other reasons included the use of contrast, technical difficulties in conducting the procedure, and the risk of procedural complications.
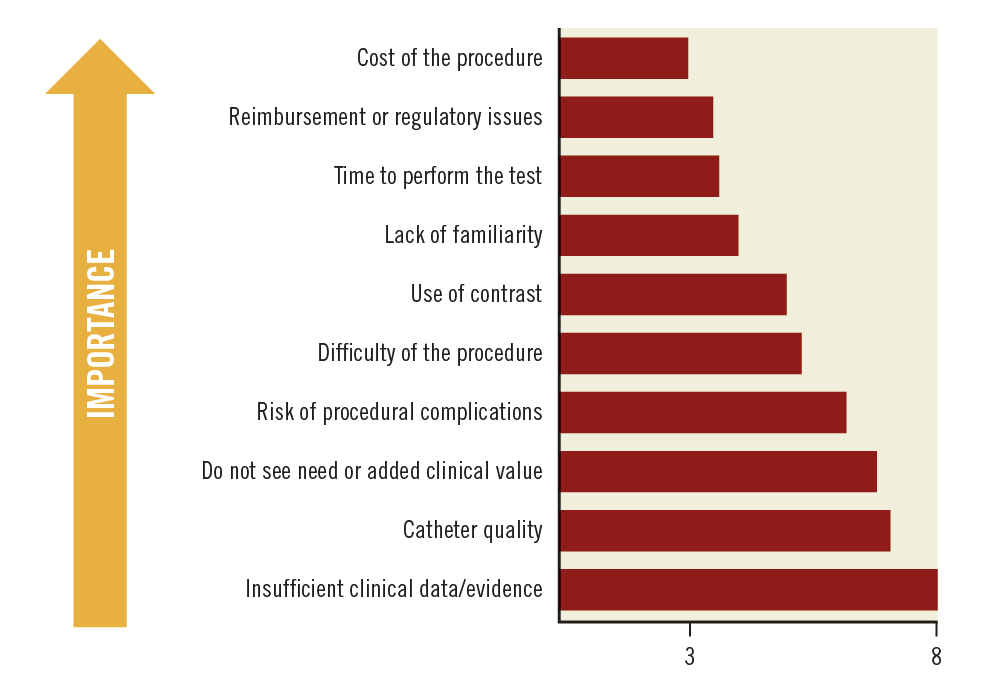
Figure 3. Key reasons for the low clinical uptake of imaging. Average scores are presented. Ranked from 1 to 10; 1=most important; 10=least important.
KEY LEARNINGS
- Cost and reimbursement are 2 major barriers to OCT usage.
- It is important to improve training and mentorship of healthcare practitioners.
- Systematic workflow with options such as the MLD MAX algorithm (morphology, length, diameter, medial dissection, apposition, expansion) has improved the acceptability of OCT12.
- Addressing the limitations of the OCT device, such as the inability to advance the catheter across tight calcified lesions, can improve the usage of OCT.
There are no specific randomised controlled trials on OCT in the SEA population. Although various studies have shown the benefits of OCT, there is a need for outcome data and regional/local data, including specific patient type/lesion subsets.
A path for the future of intravascular imaging in SEA
Not only did the experts provide their opinion regarding the future of intravascular imaging in SEA from a technological point of view, but they also suggested improvements to the acceptance and usage of imaging in SEA. For any technique, ease of use as well as the final outcome is very important. A majority of the experts felt that the availability of artificial intelligence (AI)-guided image interpretation would improve the experience and expand access to OCT usage (Figure 4). Enhanced deliverability of the catheters was also considered an added advantage. Other potential features/benefits that would improve a clinician’s experience and expand access with regard to OCT are automatic external elastic lamina detection and a streamlined workflow.
There have been innovative changes in the OCT hardware and software. Numerous innovations have been introduced in the design and delivery of imaging catheters and software. Advanced, flexible, tapered, and thin imaging catheters are available for easy navigation in tortuous vessels during OCT to capture quality images. Advanced software has enabled a wide range of options and simplified the learning curve with the use of AI, including features such as automatic detection and measurement, intuitive user interfaces, streamlined workflows, and customisable settings.
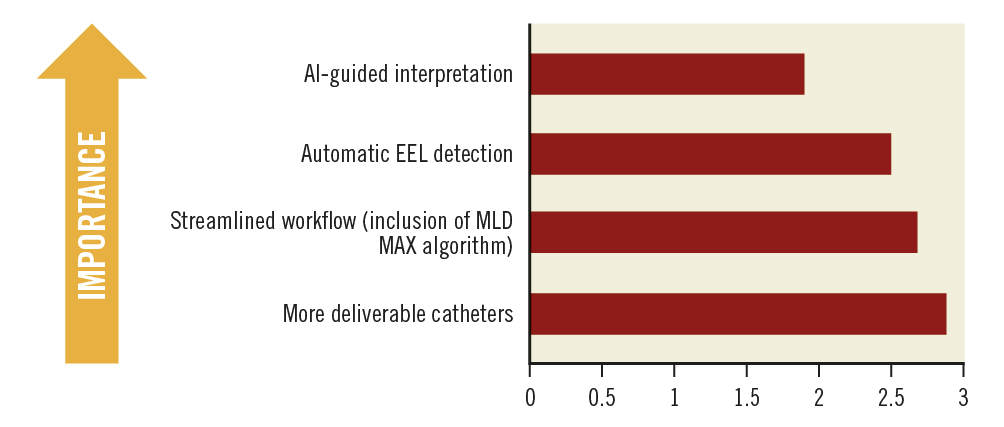
Figure 4. Importance of future features/benefits. Average scores are presented. Ranked from 1 to 4; 1=most important; 4=least important. AI: artificial intelligence; EEL: external elastic lamina; MLD MAX: morphology, length, diameter, medial dissection, apposition, and expansion
KEY LEARNINGS
- Training and mentorship have to be implemented for clinicians as well as the support staff of catheterisation laboratories.
- The availability of regional/local data has to be increased.
- The implementation of local guidelines related to OCT usage should be evaluated.
- A consensus document should be published for improved acceptance and implementation of OCT.
Final thoughts
The present study investigated the clinical point of view of SEA expert physicians on the current clinical utility of intracoronary imaging and OCT, as well as the barriers and enablers of imaging and OCT adoption, and the future of intravascular imaging in SEA. The study's main findings are the following: 1) OCT is preferred in certain lesion subsets, such as ACS, calcified lesions, left main lesions, bifurcation, and stent failure; 2) cost and reimbursement are 2 major barriers to OCT use; and, 3) the implementation of local guideline/evidence-based consensus statements, training for clinicians and support for healthcare staff, improvement of OCT software and hardware are paths to increasing future adoption of OCT in the SEA region.
Why use OCT?
In ACS patients undergoing revascularisation, OCT imaging plays a key role in identifying the culprit lesion, predicting the likelihood of no reflow, and optimising the stent13. OCT is well suited for image guidance during bifurcation stenting because of its high resolution and three-dimensional imaging ability14. OCT imaging provides extra information on calcium plate thickness that helps in selecting an appropriate method for treating calcified lesions15. Because of the high resolution of OCT, the suboptimal stent placement and structural defects of the stent are clearly visible, and this helps to reduce the likelihood of stent failure1.
OCT-guided PCI is associated with better clinical outcomes compared with angiography alone, and this has been documented in several clinical trials. The CLI-OPCI study reported that OCT-guided intervention was associated with a significantly reduced rate of cardiac death or myocardial infarction compared with angiography alone16. OCT-guided PCI has been associated with better procedural outcomes, fewer in-hospital events, and lower mortality compared with angiography-guided PCI 17. OCT-based calcium scores were used to identify calcified lesions that required plaque modification18. A lack of plaque modification in a heavily calcified lesion may lead to stent underexpansion and stent failure. Due to the complexity of the calcified lesion, OCT provided important information in pre- and post-procedural assessments, such as calcium characteristics, vessel size, calcium fracture, stent optimisation, and stent expansion. In patients with heavily calcified lesions undergoing rotablator atherectomy (RA), intravascular imaging guidance resulted in an appropriate burr-to-artery ratio, higher acute gain, and larger stent diameter when compared to angiographic guidance. Furthermore, major adverse cardiac events (MACE) and mortality were significantly reduced by intravascular imaging guidance in these patients19. Large lipid burden and thin-cap fibroatheroma (TCFA) on OCT are associated with peri-PCI myocardial infarction4. The ILUMIEN III: OPTIMIZE PCI trial reported that OCT-guided PCI facilitated superior stent expansion and technical success compared with angiography-guided PCI20. The Does Optical Coherence Tomography Optimize Results of Stenting (DOCTORS) trial reported that OCT-guided PCI was associated with greater post-procedural fractional flow reserve than angiography-guided PCI in patients with non-ST-segment elevation acute coronary syndromes21. The use of OCT has been reported to change treatment strategy. Findings from the LightLab initiative (Abbott) reported that OCT impacted procedural decision-making in 88% of PCI cases (Croce K et al. TCT CONNECT-407 Optical Coherence Tomography Influences Procedure and Vessel Preparation Decisions During Percutaneous Coronary Intervention: Insights From the LightLab Initiative. J Am Coll Cardiol. 2020;76:B175). The OPTICO-integration study has also reported a significant impact of real-time OCT coregistration with angiography on PCI strategy22. More recently, the Pan London registry reported the long-term mortality benefit of OCT-guided PCI in a large retrospective study17. The iOPTICO study has reported that real-time OCT and angiographic coregistration (OCT-ACR) during PCI led to good clinical outcomes with a MACE rate of 0.85% (Mathew R, et al. TCT CONNECT-410 Impact of Real-Time Optical Coherence Tomography-Angio Co-Registration (OCT-ACR) on Physician Decision Making During Percutaneous Coronary Intervention: A Multicenter, Prospective Study (iOPTICO study). J Am Coll Cardiol. 2020;76:B176).
Where do we stand now?
The average rate of imaging use among the experts in the present article was 51%. The average rate of IVUS (54%) performed was greater compared with OCT (46%), most likely attributable to the limited outcome data in a randomised controlled trial (RCT) in the local SEA population as well as the increased familiarity with IVUS, which has been in clinical use longer than OCT. Although there are a few studies indicating the benefits of OCT, there is a need for RCT regarding OCT use specifically in SEA. Per the experts, this would help augment the adoption of OCT in this region. On average, 9 OCT-guided procedures per month were performed by these experts in SEA. Although there is a lack of RCT related to the efficacy and safety of OCT in SEA, the experts’ interest in OCT indicates their confidence in the technique. Nearly 70% of the experts stated that good evidence is currently available regarding the benefits of OCT in the identification of TCFA, plaque rupture, and erosion, as well as different types of plaques, such as fibrous, lipid-rich, and calcific plaques4.
Despite the known benefits of OCT and experts in SEA having interest and confidence in the technique, certain barriers limit the use of OCT. The survey reported that the usage of any intracoronary imaging was low across SEA. There are no country-specific guidelines, consensus documents or societal recommendations regarding the usage of OCT in SEA. Hence, according to the experts from SEA, the existing guidelines on the usage of OCT from Western and Asian countries are followed. The clinicians should advocate for the formation of national organisations or committees to form country-specific or region-specific guidelines/consensus statements. Publishing a consensus document with regard to the use of OCT across SEA may increase its acceptance in the region. At present, there are no RCT that have investigated the usage of OCT in the SEA population. More evidence indicating beneficial outcomes with the usage of OCT would increase its adoption in SEA.
Cost is often a barrier for catheterisation laboratories. Variations in reimbursement policies/coverage across SEA, limit the use of OCT. There are variations in available healthcare resources and facilities in SEA23. A proper reimbursement policy for OCT may increase its acceptance among patients in SEA. Except in Japan, the use of OCT in Asia is hampered due to inadequate reimbursement for the procedure424.
The increased procedure time is another major barrier to the adoption of OCT. A longer time is required to perform OCT-guided PCI compared with fluoroscopy-guided PCI21. The time constraints are mainly attributed to the lack of adequate training of the staff. With adequate training, the time required to perform the procedure can be significantly reduced. Training the physicians who can subsequently train the support staff can be a good approach. A vast amount of imaging data can be acquired by performing OCT4. The lack of adequate training for the support staff in the OCT procedure as well as image interpretation is a major barrier to the adoption of OCT. The availability of an offline workstation that can be used to visualise the images and train juniors would help to improve the acceptability of OCT. User-friendliness of the device could increase the acceptability of OCT. New-generation OCT systems with software that can automate certain tasks might decrease the burden of manual work for the clinician4. Several intracoronary imaging OCT systems have been developed. The 2 most commonly employed OCT systems are the OPTIS system (Abbott Vascular) and the LUNAWAVE system (Terumo)12.
Moving forward
The future of OCT adoption in SEA depends on strategies that can address the barriers restricting its use. The use of advanced catheters and software may increase clinicians’ approval of using OCT. Recently, it has been reported that a novel AI model for automatic plaque characterisation in intravascular OCT provided good diagnostic precision in internal as well as external validation25. The AI-based software had a reported diagnostic accuracy of 97.6% in fibrous plaque, 90.5% in lipid, and 88.5% in calcium26. The median time to analysis was 21.4 seconds26. This model may decrease prejudice in image interpretation, expedite quantification of plaque composition, and may have potential in OCT-guided PCI26. Hence, with AI models in the future, the clinician would be able to capture precise images and would have the benefit of easy interpretation of the images. In addition, the MLD MAX algorithm would also be helpful in guiding treatment decisions pre- and post-PCI, resulting in optimal outcomes25. The Ultreon 1.0 software (Abbott), powered by AI, allows automatic quantification of calcification and vessel size27.
A 5E approach (i.e., evidence, education, expand capital penetration, economics, and expert consensus) could be implemented in SEA for enhanced utilisation of OCT28. Evidence could be either local or regional and should be based on decision-making strategy trials (e.g., the iOPTICO study) or country-specific large registries and specific lesion subsets. Different academic societies and organisations can help ensure that interventional cardiologists, fellows, catheterisation laboratory staff, and nurses are educated with regard to the benefits of OCT-guided PCI during the early stage of their careers. Development of the infrastructure at small or medium healthcare centres/hospitals might help to promote the adoption of OCT-guided PCI. There is a necessity to establish the cost-effectiveness of the OCT technology based on region-specific evidence. Regional- or country-specific data would also support potential reimbursement policies and, hence, could increase the adoption of OCT.
Development of new OCT systems is ongoing at the global level to increase the penetration depth, speed of OCT acquisition, and spatial resolution (to identify cellular and subcellular structures)25. Additional studies are required for the validation of new developments and their impact on clinical medicine. At present, 2 large RCT, the ILUMIEN IV28 and OCTOBER29 trials, aimed at determining the clinical impact of OCT-guided versus angiography-guided PCI are ongoing. Most of the clinical data involving OCT are generated in Western countries. The learning curve during the initial usage of OCT is quite steep and can deter its routine use. The future adoption of OCT across SEA would also depend on the availability of trained clinicians and staff to perform OCT in the catheterisation laboratories. Good image acquisition and appropriate interpretation is fundamental to OCT use, hence the importance of adequate training of clinicians and staff.
In the present article, we have provided insights into the use of OCT from a clinician’s point of view. The impact of intracoronary OCT on medical practice has been limited4. This may be due to a lack of long-term data on OCT, as research is still ongoing and outcomes are yet to be published. Given that this survey was conducted amongst frequent users of imaging and physicians who believe in the benefits of imaging-guided PCI, the survey opinion reflects an “expert opinion” and not a general prevalence.
Conclusions
To summarise, additional clinical evidence obtained through local registries, RCT, and case studies in SEA will provide further confidence to users and help to overcome some of the learning-curve challenges. Country-specific guidelines or consensus statements regarding OCT use in SEA are required for its enhanced acceptance and adoption. The future of OCT in SEA will depend on the training of staff, available evidence in the SEA population, and local guidelines or consensus documents. A survey involving experts from all countries in the SEA region would provide a better idea on the usage of OCT and the barriers limiting its use, which, in turn, would help to understand the future of intravascular imaging in the SEA region.
Acknowledgements
We would like to thank BioQuest Solutions for editorial services.
Funding
The advisory board meeting has been supported by Abbott Laboratories (S) Pte Ltd.
Conflict of interest statement
R. Kaur, M. Narang, and N.E.J. West are employees of Abbott Vascular. The other authors have no conflicts of interest to declare.

