Introduction
Pulmonary arterial hypertension (PAH) is distinguished by progressive right heart failure and subsequent premature death12. Several registry studies have reported different algorithms to predict the difference in clinical outcome among PAH patients23456. These risk stratifications are based on a comprehensive assessment including clinical features, the World Health Organization (WHO) Functional Class (FC), the six-minute walking distance (6MWD), cardiopulmonary exercise testing, biomarkers, and certain variables derived from echocardiography and right heart catheterisation (RHC). Obviously, FC is a key variable and more easily obtained from PAH patients than any other variable. Recently, an FC decrease ≥1 class served as one element of the primary endpoint in the REPLACE trial7, in line with the results from the Registry to Evaluate Early And Long-term PAH Disease Management (REVEAL registry)8, which demonstrated a better three-year survival in patients with PAH who improve from FC 3 to FC 1 or 2, compared to patients who remain in FC 3. These studies have provided a strong correlation between FC and mortality. Furthermore, the five-year analysis by the REVEAL study group reported that FC remains one of the most important predictors of future survival9. These observations reinforce the importance of continuous monitoring of FC in patients with PAH89.
Increased sympathetic nerve traffic plays an important role in advanced PAH and may be related to disease severity10. To investigate this, percutaneous pulmonary artery denervation (PADN) using radiofrequency was designed and tested in three clinical studies with small patient populations111213. While the association of PADN and significant improvements in cardiac function, haemodynamics, and exercise capacity were reported, the different responses to PADN as stratified by FC remains unclear. Accordingly, a total of 120 PAH patients who underwent PADN treatment in 11 Chinese centres and were prospectively followed-up, were included in the present analysis.
Methods
Patient population
Between March 2012 and March 2018, a total of 120 PAH patients (13 from PADN-I Phase I11, 39 from PADN-I Phase II13, and 68 between May 2014 and March 2018) who underwent PADN were analysed in the present study (Figure 1). This study was approved by the Institutional Review Board and Ethics Committee of the participating centres, and all patients provided written informed consent.
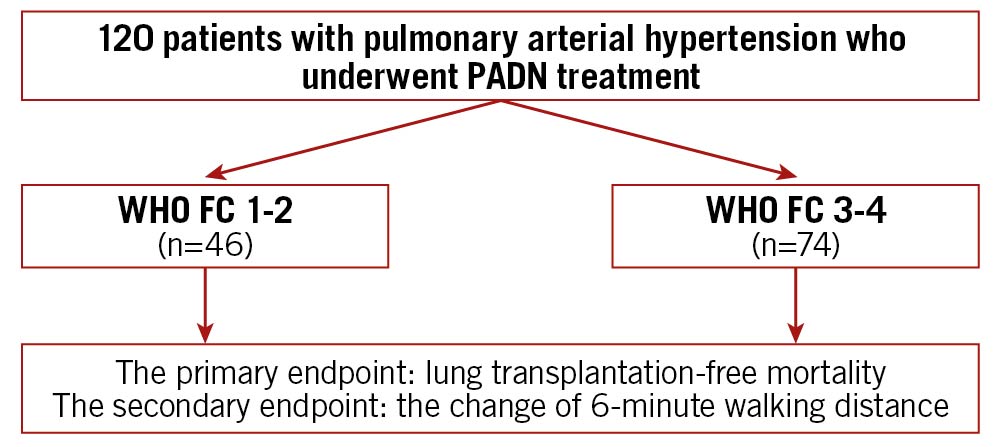
Figure 1. Study flow chart. FC: functional class; PADN: pulmonary artery denervation; WHO: World Health Organization
Inclusion and exclusion criteria
Patients with a resting mean pulmonary arterial pressure (mPAP) ≥25 mm Hg, pulmonary vascular resistance (PVR) ≥3 Wood units, and pulmonary artery occlusion pressure (PAOP) <15 mm Hg, as measured by RHC, were included. Exclusion criteria included active infection, cancer, toxin- or anorexia-induced pulmonary hypertension (PH), portal hypertension, chronic thromboembolic PH without surgical treatment, congenital heart disease, and intolerance to the PADN procedure.
Haemodynamic measurements
RHC was scheduled at rest before PADN and between 6-12 months after PADN. Right atrial pressure (RAP), right ventricular (RV) pressure, PAP, PAOP and thermodilution cardiac output were obtained with a 7 Fr flow-directed Swan-Ganz catheter. PVR ([mPAP−PAOP]/cardiac output) and pulmonary arterial compliance (PAC) were then derived. All measurements were taken at end expiration.
PADN procedure
Pulmonary artery (PA) ablation was performed using a dedicated 7 Fr temperature-sensing ablation catheter. The details of the device and the PADN procedure have been previously described1113. Briefly, PADN was performed only in the periconjunctional area between the distal main trunk and the ostial left branch (Figure 2). The following ablation parameters were programmed at each point: temperature ≥50°C, energy ≤20 W, and time at least 120 seconds for each ablation point. The procedure was interrupted for 10 seconds if the patient reported severe chest pain. Sedation was not recommended but could be used if the patient was intolerant of chest pain. Electrocardiography and haemodynamic function were monitored and recorded continuously throughout the procedure.
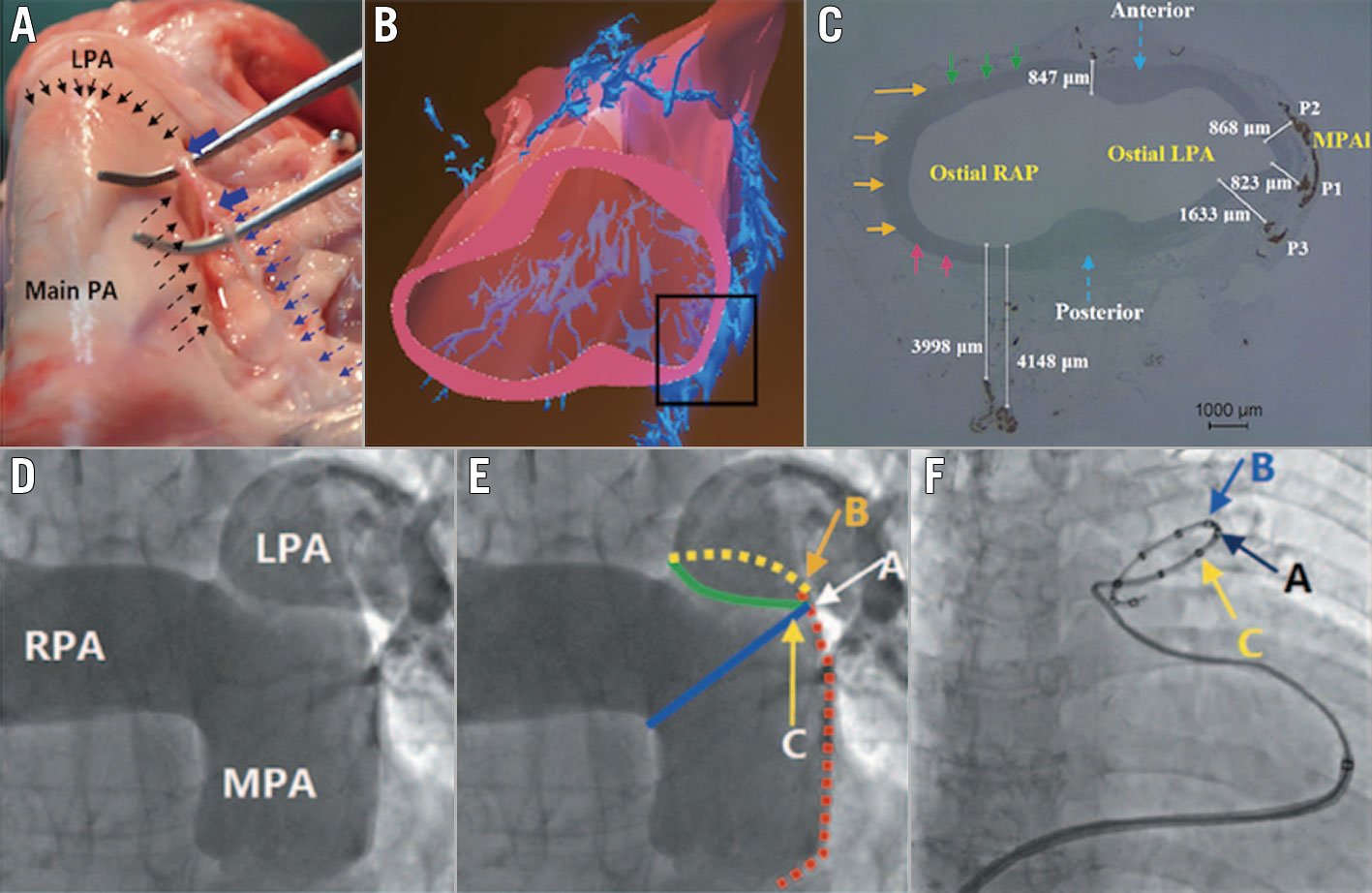
Figure 2. The mechanism and pathophysiology of PA pressure reduction from PADN. Sympathetic nerves localised in the groove (dashed black arrows in A) are parallel to the left lateral wall of the distal MPA up to the orifice of the LPA (solid black arrows); when the nerves were removed from this groove, the injured segment induced by PADN (solid blue arrows in A) became swollen. B) The three-dimensional reconstruction shows the distribution of the sympathetic nerve endings from the anterior-posterior view. C) The shortest distance (823 μm) between the nerve endings and the PA lumen at the distal PA. D) PADN was performed at three sites (A, B and C) at the conjunctional area between the distal main trunk and the ostial left branch. E) Point A: the bifurcation of the pulmonary artery; point B: the posterior wall of the ostium of the left pulmonary artery; and point C: the anterior wall of the ostium of the left pulmonary artery. F) A PADN catheter with 10 electrodes is positioned at the distal MPA. Electrodes A, B and C match with the points A, B and C shown in panel E. LPA: left pulmonary artery; MPA: main pulmonary artery; MPAI: lateral wall of the main pulmonary artery; PA: pulmonary artery; RAP: right atrial pressure; RPA: right pulmonary artery
Periprocedural medication
A 5,000-U heparin bolus was administered after the insertion of the venous sheath. PAH-target drugs (endothelin receptor antagonists, phosphodiesterase type 5 [PDE-5i] inhibitors and guanylate cyclase stimulators, and prostacyclin analogues and prostacyclin receptor agonists) were not prescribed for patients (n=52) enrolled before April 2014 and for whom there was no improvement in either the 6MWD or haemodynamics11immediately after the PADN procedure. Three months after the PADN procedure, the use of PAH-specific drugs (typically including medications that target PAH as well as basic medications such as calcium antagonists) for those 52 patients was assessed by the referring PAH physicians. For the remaining 68 patients enrolled after April 2014, PAH-targeted drugs were recommended and the strategy was left to the physician’s discretion and the patient’s economic status. Diuretics remained unchanged.
Assessment of functional capacity
Functional capacity was determined using a standard 6MWD protocol14. Dyspnoea was assessed using the Borg scale15. WHO classification of dyspnoea at rest and during exercise was recorded by a physician blinded to the study design16. Patients were classified into four classes23: Class I (symptom-free when physically active or resting), Class II (no symptoms at rest, but normal activities such as climbing the stairs, grocery shopping or making the bed cause some discomfort and shortness of breath), Class III (resting may be symptom-free, but normal chores around the house are greatly limited due to shortness of breath or feeling tired), and Class IV (symptoms at rest and severe symptoms with an activity). Baseline blood samples were obtained to measure N-terminal pro–brain natriuretic peptide (NT-proBNP) levels before each exercise test.
Echocardiographic measurements
Transthoracic echocardiography (Vivid 7; General Electric Co.) was performed prior to and at the last visit after PADN procedures and was analysed at the Nanjing Medical University Echocardiographic Laboratory (Dr Juan Zhang). Digital echocardiographic data containing a minimum of three consecutive beats (or five beats in cases of atrial fibrillation) were acquired and stored. The following parameters were measured17: RV systolic pressure, systolic PAP (sPAP), RAP, mPAP, PVR, PAC, pulmonary artery accelerating time (PAAT), and Tei index.
Follow-up
Patients were monitored in the critical care unit for at least 24 hours post-procedure. Office visits were scheduled for all patients every three months. Haemodynamic measurements, 6MWD, and echocardiographic measurements were repeated until the last visit in March 2021. For patients who were lost to follow-up or died before their scheduled visit, all measurements at the last visit were censored.
Study endpoints and definitions
The primary endpoint was lung transplantation-free mortality at the end of follow-up (cut-off date March 2021). Secondary endpoints included lung transplantation and PAH-related rehospitalisation. A ≥15% decrease in 6MWD from baseline, confirmed by a second 6MWD performed on a different day within 14 days, was an additional secondary endpoint. PADN procedural haemodynamic success, defined as a reduction in mPAP immediately after PADN by ≥10%, without the occurrence of any intraprocedural complications, was also calculated.
Statistical analysis
According to WHO FC classifications23, patients were grouped into either FC 1-2 or FC 3-4.
Continuous variables were expressed as mean±standard deviation (SD). Normality was examined using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Data having unequal variances were transferred to log data. Differences in continuous variables between two groups were analysed with Student’s t-test. Categorical variables between groups were compared by using Fisher’s exact or chi-square test, as appropriate. Event-free survival rate was estimated using the Kaplan–Meier method. Univariate variable analysis was used to analyse the correlation of individual variables with the primary endpoint. Any variable with a p-value <0.05 was input into a multivariate analysis of mortality. Statistical significance was defined as a two-sided p-value of <0.05. All analyses were performed with SPSS 24.0 (SPSS Institute Inc.).
Results
Patient population and baseline clinical characteristics
Of a total of 120 patients (mean age of 40.7 years), 46 (38.3%) patients were in the FC 1-2 group and 74 (61.7%) were in the FC 3-4 group (Table 1). Idiopathic PAH (IPAH) was more frequently seen in the FC 3-4 group (67.6%), compared to the FC 1-2 group (39.1%, p=0.003) (Figure 3).
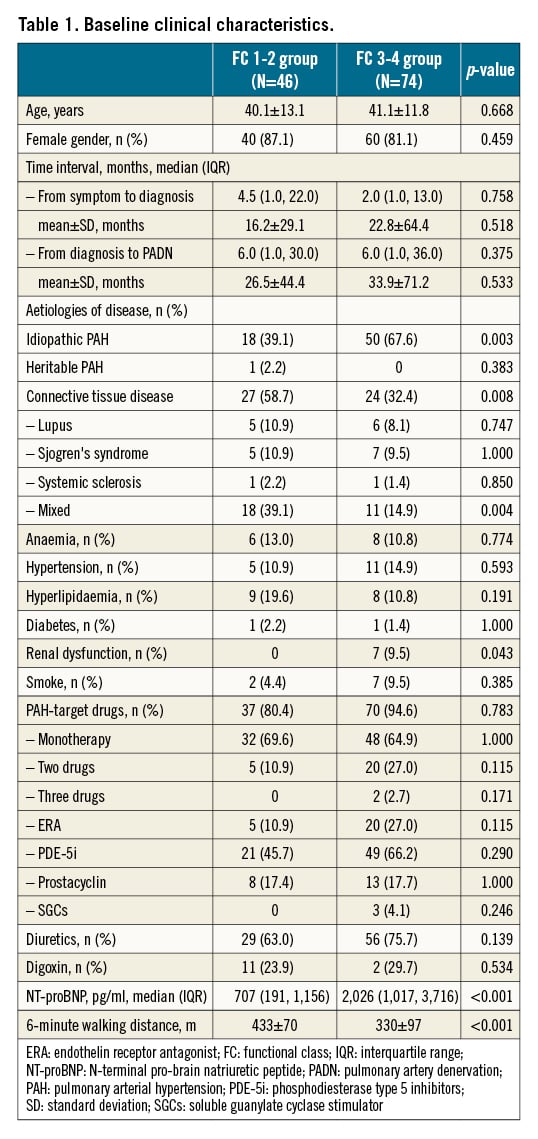
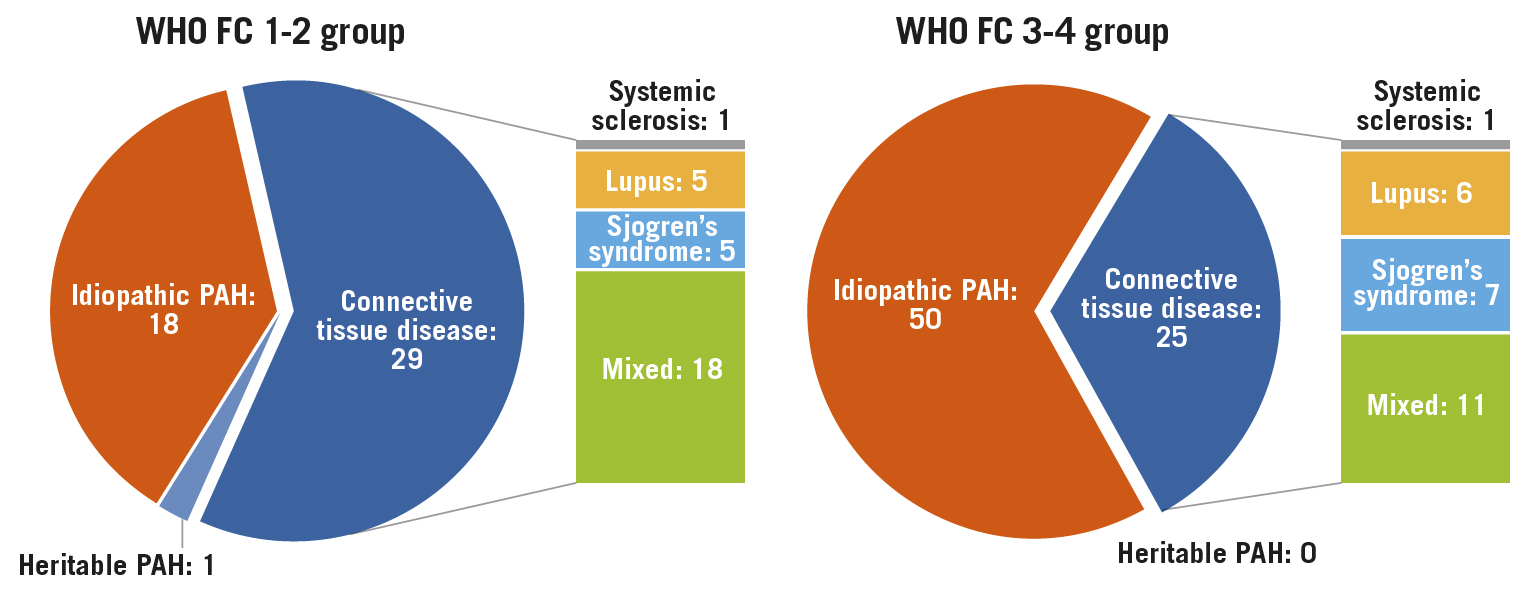
Figure 3. Patient characteristics according to WHO Functional Class for PAH. PAH: pulmonary arterial hypertension
The median time interval from the onset of symptoms to diagnosis of PAH was 4.5 (interquartile range [IQR], 1.0-22.0) months in the FC 1-2 group, a non-significant difference to 2.0 (IQR, 1.0-13.0) months in the FC 3-4 group (Table 1). The median time interval from the diagnosis of PAH to performance of PADN was also comparable between the two groups. FC 3-4 group was characterised by more common renal dysfunction (9.5%) and elevated NT-proBNP (2,026 pg/ml of median value), compared to 0% (p=0.043) and 707 pg/ml (p<0.001) in the FC 1-2 group, mirroring the decreases of 6MWD (330±97 m vs 433±70 m, p<0.001), respectively. PAH-specific drugs were equally used in the two groups, either as monotherapy or in combination. Monotherapy was the dominant strategy.
Worsening of cardiac function and haemodynamicS over functional class
As measured by echocardiography, patients in the FC 3-4 group had a large amount of pericardial effusion (median 2.0 mm vs 0 mm, p=0.004), enlarged right atrium (RA) (56.9±10.9 mm vs 50.6±9.2 mm, p=0.003), and RV failure (47.5±9.0 mm vs 42.8±7.8 mm, p=0.007, Table 2), compared to those in FC 1-2 group, respectively. Tricuspid annual plane systolic excursion (TAPSE) was 12.49±2.19 mm in the FC 3-4 group, significantly lower than in the FC 1-2 group (14.0±2.63 mm, p=0.004), along with the increased RV Tei index in the FC 3-4 group (2.05±0.88 vs 0.49±0.12, p=0.015).
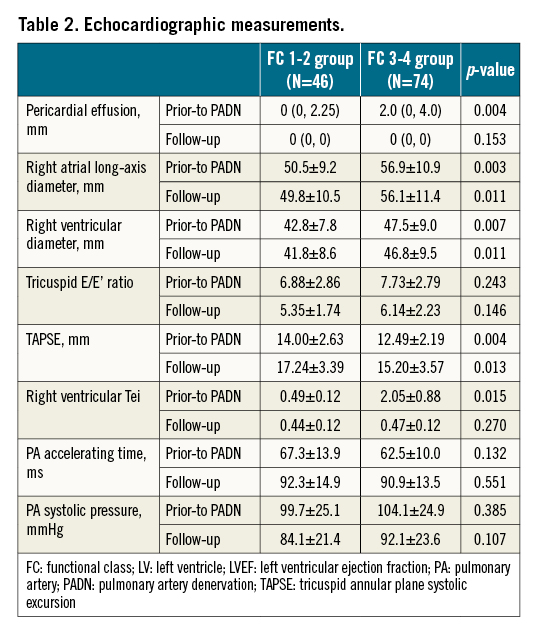
Follow-up data of right cardiac catheterisation showed a decrease in cardiac output, cardiac index and PAC, but an increase in PAP, PVR and RAP in both groups regardless of grade, FC 1-2 or FC 3-4 (Table 3).
Safety and efficacy of PADN procedures
PADN was successfully performed in all patients. During the procedures, almost half of the patients complained of chest pain, but sedation was only required for one patient (Table 4). After the procedures, one patient had a haematoma at the access site (right femoral vein). There was no PA perforation.
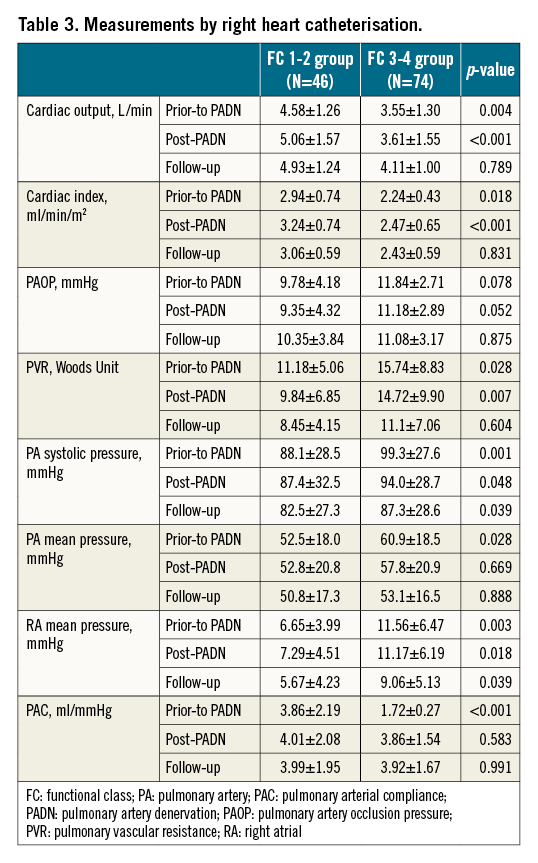
Echocardiographic parameters were measured in 38 (82.6%) FC 1-2 patients and 59 (77.6%) FC 3-4 patients through a median of 4.8 years follow-up (Table 2). Cardiac index, TAPSE, Tei index, PAAT, PVR, RV or PA pressures, and pericardial effusion were all significantly and equally improved by PADN treatment.
Immediately after the completion of PADN procedures, the reduction of sPAP or mPAP was around 5% in the FC 3-4 group (Table 3). At 6-12 months post-procedure, the total reduction in sPAP or mPAP was non-significant between the two groups (Table 3, Figure 4). However, the increase in cardiac output in the FC 3-4 group was +19.7%, compared to +12.1% in the FC 1-2 group (p=0.043), leading to more profound reduction in PVR in the FC 3-4 group (-22.8% vs -11.2%, p=0.033).
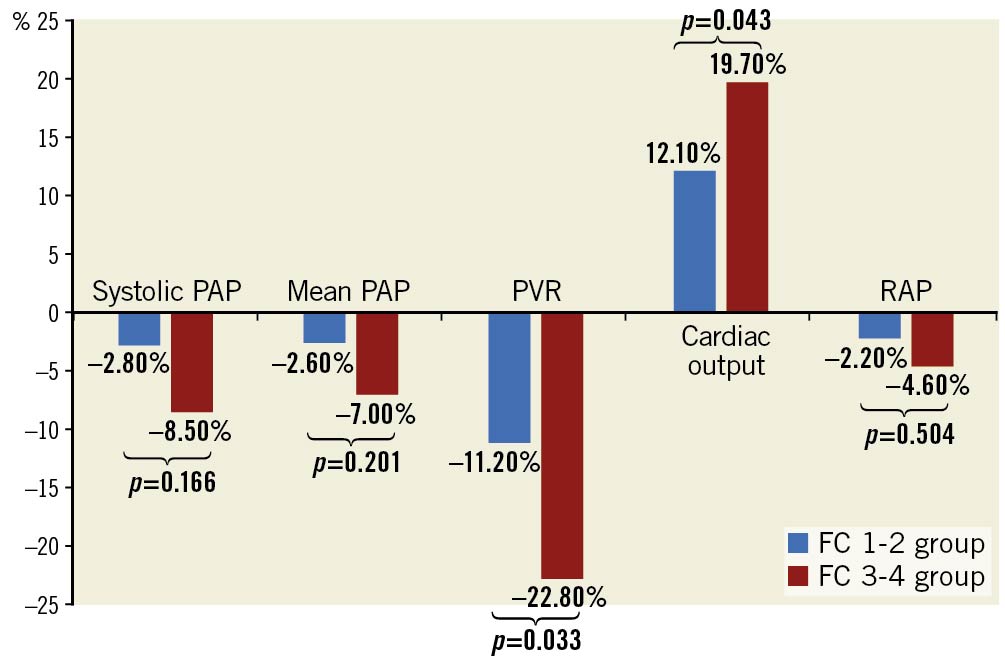
Figure 4. Percentage reduction in haemodynamic measurements at 6-12 months after PADN. PADN: pulmonary artery denervation; PAP: pulmonary arterial pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure
PAH-specific medications after discharge
After discharge, pulmonary arterial hypertension (PAH)-specific drugs were prescribed for 68 patients enrolled after March 2014. Of 52 patients who did not receive PAH-target drugs immediately after PADN, 44 continued PAH-specific medications at three months after procedure, leading to a total of 112 (93.3%) patients undergoing treatment using target drugs, without statistical difference between the two groups (p=0.117). Monotherapy was still the primary treatment in the two groups, with PDE-5i mostly being used.
Clinical outcomes
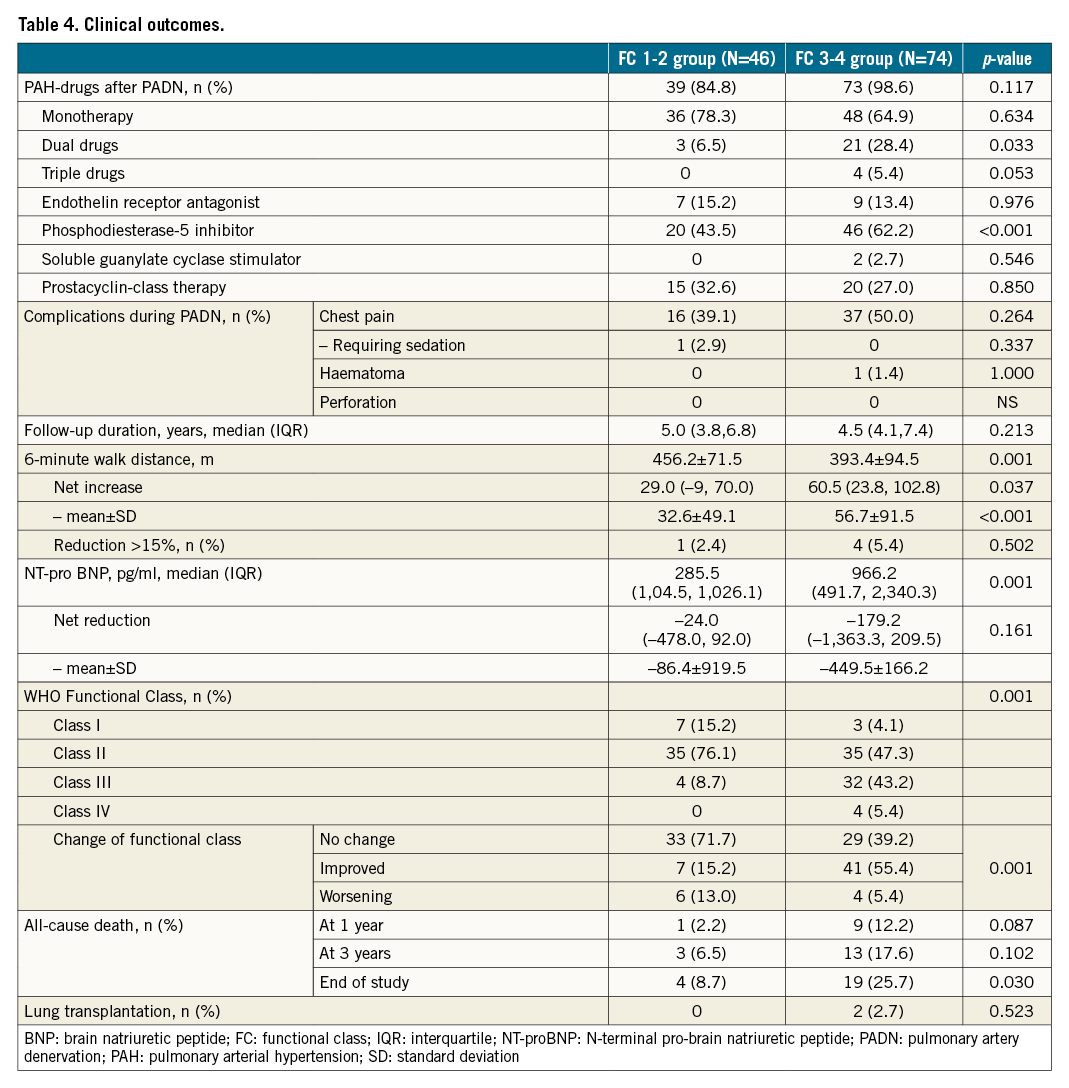
The median duration of follow-up was 5.0 years in the FC 1-2 group, compared with 4.5 years in the FC 3-4 group (p=0.213) (Table 4).
Exercise capacity
The net increase of 6MWD was 32±49 m in the FC 1-2 group and 56±92 m in the FC 3-4 group (p=0.035) (Table 4), along with the greater net reduction of NT-proBNP in the FC 3-4 arm (p=0.003). At the end of follow-up, five (4.2%) patients had a reduction of >15% in 6MWD, with one (2.4%) in the FC 1-2 group and four (5.4%) in the FC 3-4 group (p=0.502). According to the WHO Functional Class at the end of follow-up, PADN was associated with a greater improvement in the FC 3-4 group (55.4%), compared to 15.2% in the FC 1-2 group (p=0.001).
Mortality
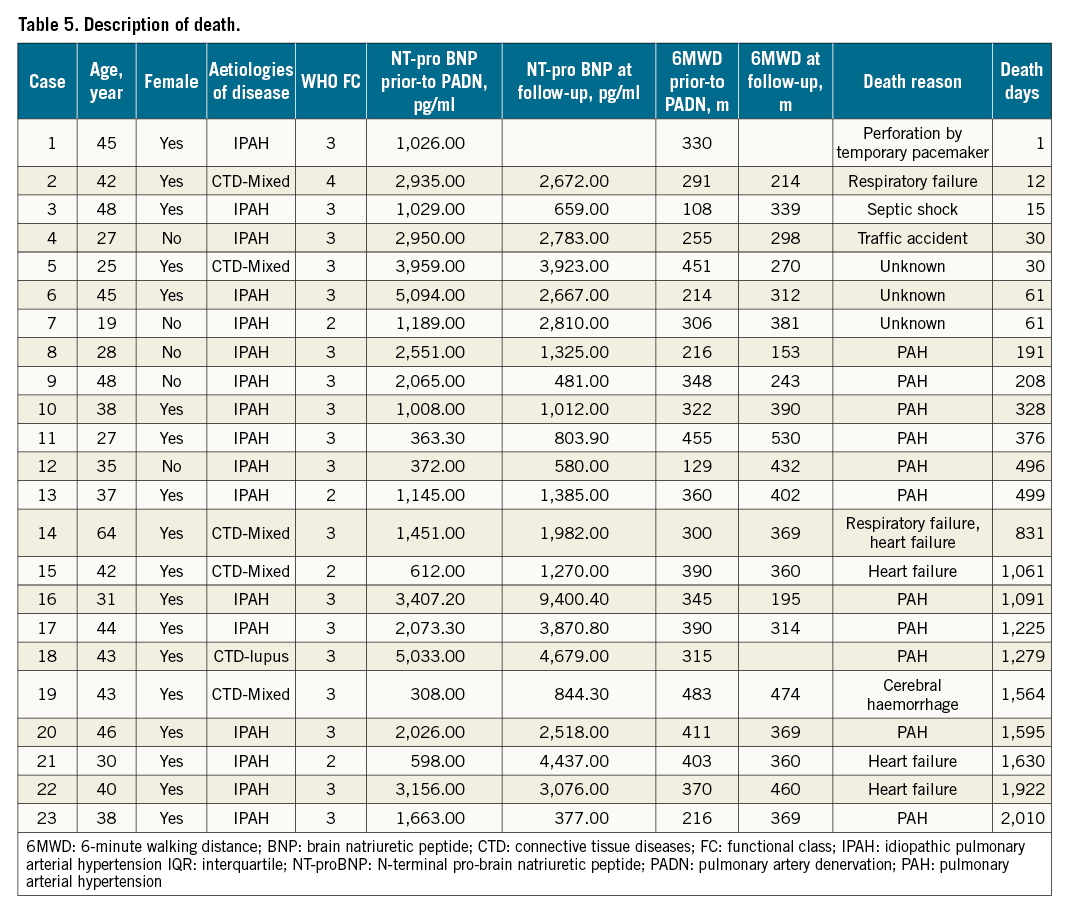
During follow-up, 23 (19.2%) patients died (Table 4, Figure 5A), mostly in the FC 3-4 group (n=19, 25.7%), compared to 8.7% (n=4) in the FC 1-2 group (Kaplan-Meier log-rank analysis, p=0.033; hazard ratio [HR] 0.330, 95% confidence interval [CI]: 0.112-0.970; p=0.044). The one-year (Figure 5B) and three-year (Figure 5C) mortality in the FC 1-2 group were 2.2% and 6.5%, respectively, compared to 12.2% (Kaplan-Meier log-rank analysis, p=0.087; HR 0.170, 95% CI: 0.022-1.345; p=0.093) and 17.6% (Kaplan-Meier log-rank analysis, p=0.102; HR 0.334, 95% CI: 0.098-1.208; p=0.096, Table 4) for the FC 3-4 group, respectively. Two high-risk patients underwent lung transplantation. The attribution of death is shown in Table 5.
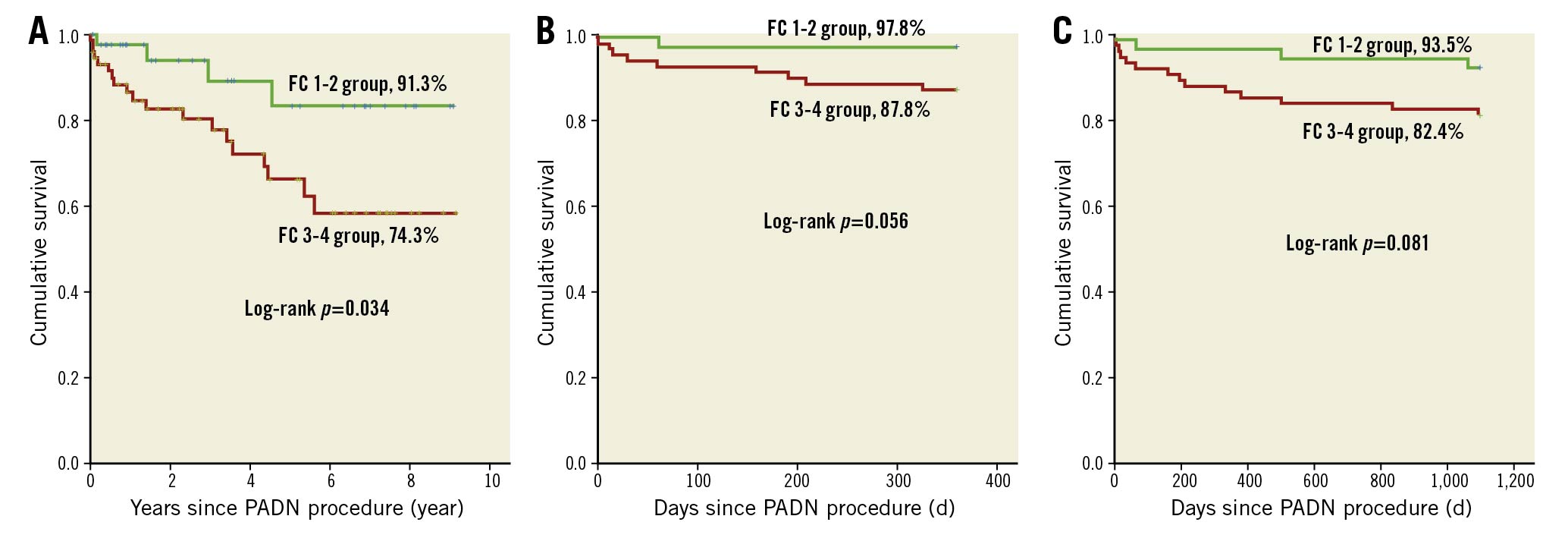
Figure 5. Kaplan-Meier survival between FC 1-2 and FC 3-4 groups. A) At the end of the study; B) at one year; C) at three years. FC: functional class; PADN: pulmonary artery denervation
Discussion
This consecutive study with a large sample size provides, for the first time, the differential responses to PADN at long-term follow-up among PAH patients stratified by WHO Functional Class. The major findings are: 1) the functional class is capable of reliably identifying high-risk PAH patients; 2) PADN is associated with significant improvements in cardiac function, haemodynamics and exercise capacity, particularly among patients in FC 3 and 4; and 3) notably, PADN results in favourable long-term survival at a median of 4.8 years follow-up, especially in patients with FC 3 or 4.
The causes of mortality in patients with PAH are multifactorial, including severity or progression of disease and underlying aetiologies461819202122. For example, patients with PAH associated with congenital heart disease (CHD-PAH), which was excluded from our analysis, live longer than those with connective tissue disease-associated PAH (CTD-APAH)1921. As such, the mortality rate in the current study might be higher than reports including CHD-PAH.
FC as a key element of risk scores was initially proposed by the REVEAL study group19 and was also included in the modified REVEAL 2.0 risk score calculator19. The association of FC with three- and five-year mortality has been confirmed by two observational studies89. In our study, we found that patients with FC 3 or 4 had more IPAH and renal dysfunction, deterioration of cardiac dysfunction, and severe remodelling of PA, leading to a loss in exercise capacity. All these results indicate the important role of FC in identifying high-risk patients and predicting long-term clinical outcome for PAH patients.
PADN treatment was introduced based on the theory that increased sympathetic nerve traffic plays an important role in advanced PAH and may be related to disease severity10. Our initial pilot study11 showed a significant net increase in 6MWD (>+100 m) and a decrease in serum NT-proBNP and the RV Tei index in 13 PAH patients who were refractory to PAH-specific drugs at three months after PADN. A subsequent registry study13 reported an increase of 94 m in 6MWD at one-year follow-up after a PADN procedure among patients with different aetiologies of PH. For patients with combined pre- and post-capillary PH23, the least mean square increase in 6MWD was 63 m, similar to the 71 m difference in patients with residual PH after pulmonary endarterectomy24. The differences in aetiology, sample size, and follow-up duration may be the underlying mechanisms for the different net gain of 6MWD. However, the consistent finding from those studies was the simultaneous significant improvement of cardiac function and haemodynamics after PADN. Echocardiography (resting and exercise) represents a key non-invasive imaging test in the diagnostic-prognostic-therapeutic PAH algorithm25. Regarding the heart structure, a consistent response to PADN was found in the present study, but reduction in the RV Tei index was more significant in FC 3-4 patients. However, the 3 mm increase in TAPSE and >25 ms increase in PAAT, as well as the significant reduction of PAP, PVR and the increase of PAC measured by RHC in the two groups, indicated the benefits of PADN treatment for PAH patients.
In 982 PAH patients with Functional Class III, Barst et al8 reported a one-year mortality rate of 17.1%, similar to 14.4% in a study (including FC 3 and 4) by Farber et al9, which was comparable with 12.2% in our study (Table 4). Furthermore, the REVEAL registry9 also reported a one-year mortality rate (5.1%) in patients with FC 1 and 2, similar to the 2.2% in our analysis. These analyses indicated that the benefits of PADN at one-year follow-up for PAH patients were not achieved across all FC groups.
PAH patients have a very poor survival rate at long-term (≥3 years) follow-up26. The mean mortality rate at three years since diagnosis was 35.8%8272830 (Supplementary Figure 1), varying from 11.8%29 to 46%30 across studies, with a much higher rate in high-risk patients (65.7%)30. This difference may be related to sample size, definition, and aetiologies (with- versus without- CHD). In the REVEAL registry, Barst et al8 reported 44.1% three-year mortality among patients in FC 3, similar to the 39.5% in patients in FC 3 and 4 reported by Farber et al9; two times higher than the 17.6% in our results. On the other hand, among patients with FC 1 and 2, the three-year mortality (18.2%) from the REVEAL registry9 was almost triple the 6.5% among our patients. The difference in five-year mortality between the REVEAL registry study and ours widened even further.
Limitations
The present analysis has some limitations. First, the lack of a control group blocks the direct comparison of all parameters and endpoints. However, the comparison of before- and after-PADN may provide evidence showing the improvements in cardiac function, haemodynamics, and exercise due to PADN treatment. Second, both echocardiography (at different time intervals after PADN) and RHC (6-12 months after PADN) were not performed at a fixed point in time. One should be very careful to extrapolate those results into long-term measurements. But the results are clearly correlated with the clinical benefits after PADN. Third, monotherapy after PADN was prescribed for most patients. This may carry the bias of analysis. However, the PAH-specific medications were equally distributed among patients stratified by risk scores, which could minimise the differential effects of target drugs on mortality. Fourth, some patients did not complete the five-year follow-up. As a result, we provided the results at a median follow-up duration (4.8 years), which should enhance our conclusions. Sixth, the population is still small and needs to be expanded to more centres, races and ages, and needs to be developed into meta-analyses and randomised clinical trials with control groups.
Conclusions
PADN is associated with significant improvements in survival at a median duration of 4.8 years of follow-up, especially in patients with FC 3 and 4. Further randomised studies using sham control are warranted to confirm the efficacy of PADN.
Impact on daily practice
This novel study elucidated the differential responses to PADN in a long-term follow-up among patients with Group 1 PAH, stratified by their WHO functional classes. PADN considerably improved survival, haemodynamics and cardiac functions at long-term follow-up, particularly in FC 3-4 patients.
Acknowledgments
We thank Dr. Ling Lin and her clinical follow-up team for their continuous data collection and remote monitoring through the whole study period. We would like to deeply thank all coordinators at each centre for their patient follow-up.
Funding
This study was funded by grants from Nanjing Health Bureau and the National Science Foundation of China (Funding number: NSFC 91639303 and NSFC 81770441).
Conflict of interest statement
S.L. Chen is the inventor of and holds patents for a pulmonary artery denervation device, but he is not the owner. The other authors have no conflicts of interest to declare.
