Introduction
Coronary angiography (CA) is routinely used to assess the extent and degree of coronary artery disease (CAD) and guide percutaneous coronary intervention (PCI). However, CA only provides a two-dimensional luminogram and has inherent limitations in evaluating the vessel wall pathology and lumen stenosis1, which makes it inadequate for strategising PCI procedures with precision. A growing body of evidence suggests that intracoronary imaging, especially intravascular ultrasound (IVUS) and optical coherence tomography (OCT), is superior to CA in guiding PCI234. Three-dimensional OCT imaging, with its higher spatial resolution, provides unique insights into plaque morphology and procedure-related complications and is more valuable in managing complex lesion subsets, such as acute coronary syndrome (ACS), bifurcation, and calcified lesions456.
Angiographic coregistration (ACR; the OPTIS integrated system [OPTISi; Abbott]) is a new technology that facilitates real-time coregistration of OCT images with the angiogram in the catheterisation laboratory7. This enables a matched side-by-side view of OCT and angiography, eliminates the need for the currently used stand-alone, mobile OCT carts, and provides accurate OCT measurements, which might improve short- and long-term clinical outcomes post-PCI78. The OPTISi system is being increasingly used in busy catheterisation laboratories in South Asia. However, clinical outcomes in such settings are sparsely reported. The present study evaluated the impact of real-time OCT and ACR on physician decision-making in a real-world scenario. The outcomes of the study could further help PCI optimisation in South Asia.
Methods
Study design and population
This was a multicentre, prospective, investigator-initiated, non-randomised, observational study conducted between November 2018 and March 2020 across nine centres in South Asia. Consecutive patients scheduled for OCT-guided PCI who met the inclusion criteria and were willing to participate in the study were enrolled. The detailed protocol and patient flow are provided in Supplementary Appendix 1 and Supplementary Figure 1, respectively. The study enrolled adults (aged ≥18 years) with angiographic diameter stenosis >70% in at least one native coronary artery. Patients with extreme angulation (>90°) or excessive vessel tortuosity, renal insufficiency (estimated glomerular filtration rate <50 mL/min/1.73 m2) or any other severe medical condition interfering with patients’ safety or study results, contraindications to dual antiplatelet therapy up to 1 year, and life expectancy of less than 1 year were excluded from the study.
Procedures
The study was initiated after obtaining ethical committee approvals at the respective institutes. Angiographic lesion complexity was classified according to the American Heart Association (AHA) consensus statement. The patients underwent conventional angiography followed by preprocedural OCT imaging and ACR. All study procedures were carried out using intracoronary OCT imaging by clinicians experienced in PCI. OCT was performed with a Dragonfly OPTIS imaging catheter (Abbott). The OCT catheter was introduced using a standard 0.014 inch guidewire, and image acquisition was done after the administration of intracoronary nitrates (100 μg). Blood was cleared from the vessel by injecting contrast medium, either manually or using an automated power injector. OCT was performed at a frame rate of 180 frames/second and with a frame density of 5 frames/mm. Real-time ACR (semiautomatic) was performed with the OPTIS integrated system to coregister the angiogram recorded during the OCT pullback with the OCT image7. The pre- and post-PCI procedural strategies were prospectively assessed following angiography, OCT and ACR. The interventional strategy, defined by the clinician, was recorded by an independent research assistant in real time following (a) CA, (b) OCT imaging and (c) ACR. As the OCT console is usually equipped to provide the ACR details after performing OCT, the investigator was blinded to the ACR details until the OCT-only-based strategy had been documented. The following preprocedural variables were collected as a part of the clinician’s interventional strategy: need for predilatation, intended need for lesion debulking by means of scoring/cutting balloons and rotablation, vessel reference diameter, intended stent length and diameter, device landing zone, and stenting strategy (i.e., 1- vs 2-stent strategy). After documentation of the preprocedural variables, PCI was performed by the clinician according to standard techniques and current guidelines. Post-PCI OCT imaging was performed with ACR.
The need for further optimisation was based on the following variables: stent underexpansion, malapposition, geographic miss, edge dissection, and tissue prolapse. All the angiographic and OCT images were independently reviewed at the designated core lab (Indian Cardiology Research Foundation). During the study, the core lab trained all sites on image capturing, the transfer of images for assessment and also provided real-time feedback to sites. Data related to study enrolment and follow-up are summarised in Figure 1A and Figure 1B. Overall, 28 patients were excluded because of inadequate OCT image quality for analysis. All enrolled patients were followed up, either telephonically or clinically, at 6 months and 1 year. Details about the patient’s well-being and any hospitalisation/revascularisation after the initial PCI were recorded at follow-up.
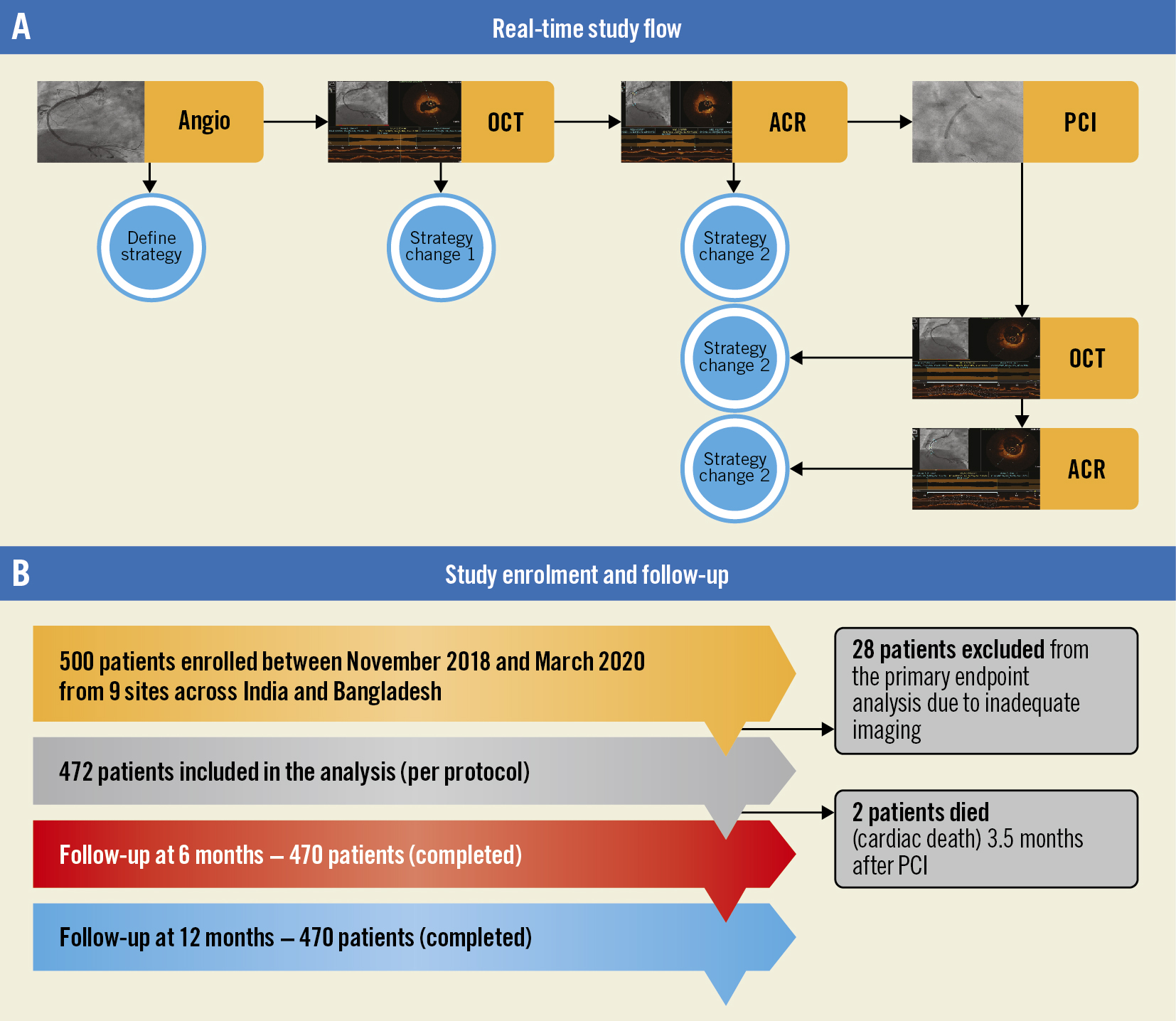
Figure 1. Study flow, enrolment and follow-up. A) Study flowchart. B) Study enrolment and follow-up. ACR: angiographic coregistration; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Endpoints
The primary endpoint was a change in the procedural strategy pre- and post-PCI with ACR. Pre-PCI strategy change involved the need to change the intended stent length and size, stenting strategy, and/or intended device landing zone. Post-PCI strategy change involved the need for additional interventional procedures for PCI optimisation after identification of stent underexpansion, malapposition, geographic miss, edge dissections and/or tissue prolapse.
The secondary endpoints included (a) procedural outcomes with ACR-guided PCI in the entire study population; (b) the rate of major adverse cardiovascular events (MACE; composite of cardiac death, myocardial infarction [MI], and target lesion revascularisation [TLR]) at 6 months and 1 year with ACR-guided PCI; (c) total change in the procedural strategy pre-PCI with OCT imaging versus angiography; and (d) time to conduct OCT and ACR (pre- and post-PCI) and contrast volume used.
Definitions
Optimal stent expansion was defined as a minimum stent area >80% of the average reference lumen area. Significant stent malapposition was defined as a strut-to-vessel wall distance of ≥400 μm extending for ≥1 mm (measured from the endoluminal strut surface to the lumen contour)1. Significant edge dissection was defined as a flap meeting either one or more of the following criteria: involving >60° of the vessel circumference, >2 mm in longitudinal extension, and the involvement of media or deeper layers1. Significant tissue protrusion was defined as intrastent tissue protrusion/thrombus reducing the residual lumen area to <4 mm2 or a reduction in the flow area of >20%9. Angiographic success was defined as <30% residual stenosis and Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow. Procedural success was defined as angiographic success without major procedural or in-hospital complications (death, MI, emergent bypass surgery, or repeat PCI). All these events were adjudicated by the institutional review board.
Statistical analyses
No formal sample size was calculated as it was a single-arm, observational study. All the categorical variables are presented as numbers and percentages and continuous data as mean and standard deviation (SD).
Results
Baseline characteristics
Between November 2018 and March 2020, a total of 500 patients with 511 lesions underwent PCI with OCT assessment. Of them, 472 patients (483 lesions), who had all parameters related to OCT data, were considered for the final analysis. The mean age of the study population (n=472) was 57.8±10.4 years, and 16.6% were females. The major coronary risk factors were systemic hypertension (58%) and diabetes mellitus (52%). About two-thirds of patients presented with ACS. Baseline clinical characteristics are summarised in Table 1.
Table 1. Baseline characteristics.
| Demographics | Patients (n=472) |
|---|---|
| Age, years (mean±SD) | 57.8±10.49 |
| Female | 78 (16.6) |
| Male | 394 (83.4) |
| Medical history | |
| DM | 245 (52.1) |
| Dyslipidaemia | 55 (11.7) |
| Hypertension | 274 (58.1) |
| FH CAD | 108 (22.9) |
| Previous PCI | 83 (17.6) |
| Previous CABG | 22 (4.7) |
| Previous MI | 33 (7.0) |
| HF | 10 (2.1) |
| Smoking | 42 (9.0) |
| Alcohol | 36 (7.4) |
| Renal disease | 7 (1.5) |
| Bronchial asthma | 8 (1.7) |
| Indication for PCI | |
| Unstable angina | 117 (24.8) |
| Recent STEMI | 95 (20.0) |
| Stable angina | 83 (17.6) |
| NSTEMI | 76 (16.1) |
| Asymptomatic positive stress test | 37 (7.4) |
| Others | 64 (13.7) |
| All data are presented as n (%) unless otherwise indicated. CABG: coronary artery bypass grafting; CAD: coronary artery disease; DM: diabetes mellitus; FH: familial hypercholesterolaemia; HF: heart failure; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction | |
Lesion characteristics and procedural details
Table 2 summarises the baseline vessel/lesion characteristics and procedural details. The majority of the lesions were found in the left anterior descending coronary artery (62%). In terms of lesion characteristics, bifurcation lesions were the most common (14.3%), whereas moderate or severe calcification was observed in 11.6% of lesions. About 87% of lesions were classified as type B and type C lesions (American College of Cardiology/AHA classification).
Table 2. Lesion characteristics and procedural details.
| Lesion characteristics (n=483 lesions) | |||
|---|---|---|---|
| Vessels treated | LAD | 300 (62.1) | |
| RCA | 111 (22.7) | ||
| LCx | 43 (8.9) | ||
| LM | 25 (5.2) | ||
| RI | 4 (0.8) | ||
| Lesion characteristics | Ostial lesion | 54 (11.2) | |
| Thrombus | 33 (6.8) | ||
| CTO | 6 (1.2) | ||
| Bifurcation lesion | 69 (14.3) | ||
| Calcification | 232 (48) | ||
| Mild | 171 (35.4) | ||
| Moderate | 34 (7.0) | ||
| Severe | 27 (5.6) | ||
| Lesion length, mm (mean±SD) | 25.5±12.0 | ||
| Long lesions (>20 mm) | 288 (60.0) | ||
| Preprocedure diameter stenosis, % (mean±SD) | 85.0±8.1 | ||
| ACC/AHA classification | A | 62 (12.8) | |
| B1 | 191 (39.5) | ||
| B2 | 108 (22.4) | ||
| C | 122 (25.3) | ||
| TIMI flow | 1 | 34 (7.0) | |
| 2 | 186 (38.5) | ||
| 3 | 263 (54.5) | ||
| Time taken for OCT and ACR | Preprocedure | Post-procedure | |
| OCT image acquisition, mins (mean±SD) | 6.6±4.0 | 10.7±1.1 | |
| Assessment of OCT, mins (mean±SD) | 3.6±2.2 | 8.3±1.1 | |
| Assessment of ACR, mins (mean±SD) | 3.9±2.3 | 7.3±0.9 | |
| All data are presented as n (%) unless otherwise indicated. ACC/AHA: American College of Cardiology/American Heart Association; ACR: angiography coregistration; CTO: chronic total occlusion; LAD: left anterior descending artery; LCx: left circumflex; LM: left main; OCT: optimal coherence tomography; RCA: right coronary artery; RI: ramus intermedius; SD: standard deviation; TIMI: Thrombolysis in Myocardial Infarction | |||
Pre-PCI strategy change
Preprocedural OCT resulted in a change in PCI strategy in 80% of lesions, which included the need for lesion preparation (25%) and change in stent length (53%), stent diameter (36%), stenting strategy (3%) and device landing zone (61%). The subsequent use of ACR further altered the treatment strategy in 34% of lesions, which included the need for lesion preparation (15%) and change in stent length (10%), stent diameter (5%) and device landing zone (16%) (Figure 2A, Figure 2B). Overall, preprocedural ACR with OCT resulted in a strategy change in 86% of lesions. The mean (±SD) stent lengths as assessed by CA, OCT and ACR were 29.6±12.2 mm, 32.5±13.3 mm and 33.0±13.4 mm, respectively. The mean (±SD) stent diameters as assessed by CA, OCT and ACR were 2.95±0.43 mm, 3.00±0.45 mm and 3.00±0.45 mm, respectively. The differences in the mean stent length were statistically significant when CA versus OCT (p<0.0001), CA versus ACR (p<0.0001) and OCT versus ACR (p=0.0006) were compared. The differences in the mean stent diameter were statistically significant when CA versus OCT (p=0.0004) and CA versus ACR (p=0.0003) were compared.
Further, the need for debulking was evident in a higher number of cases following OCT-ACR (8.5%) compared to CA (5.4%). An overall strategy change was noted in 17 (3.5%) cases following OCT-ACR; debulking was carried out by means of scoring/cutting and/or rotablation in these cases.
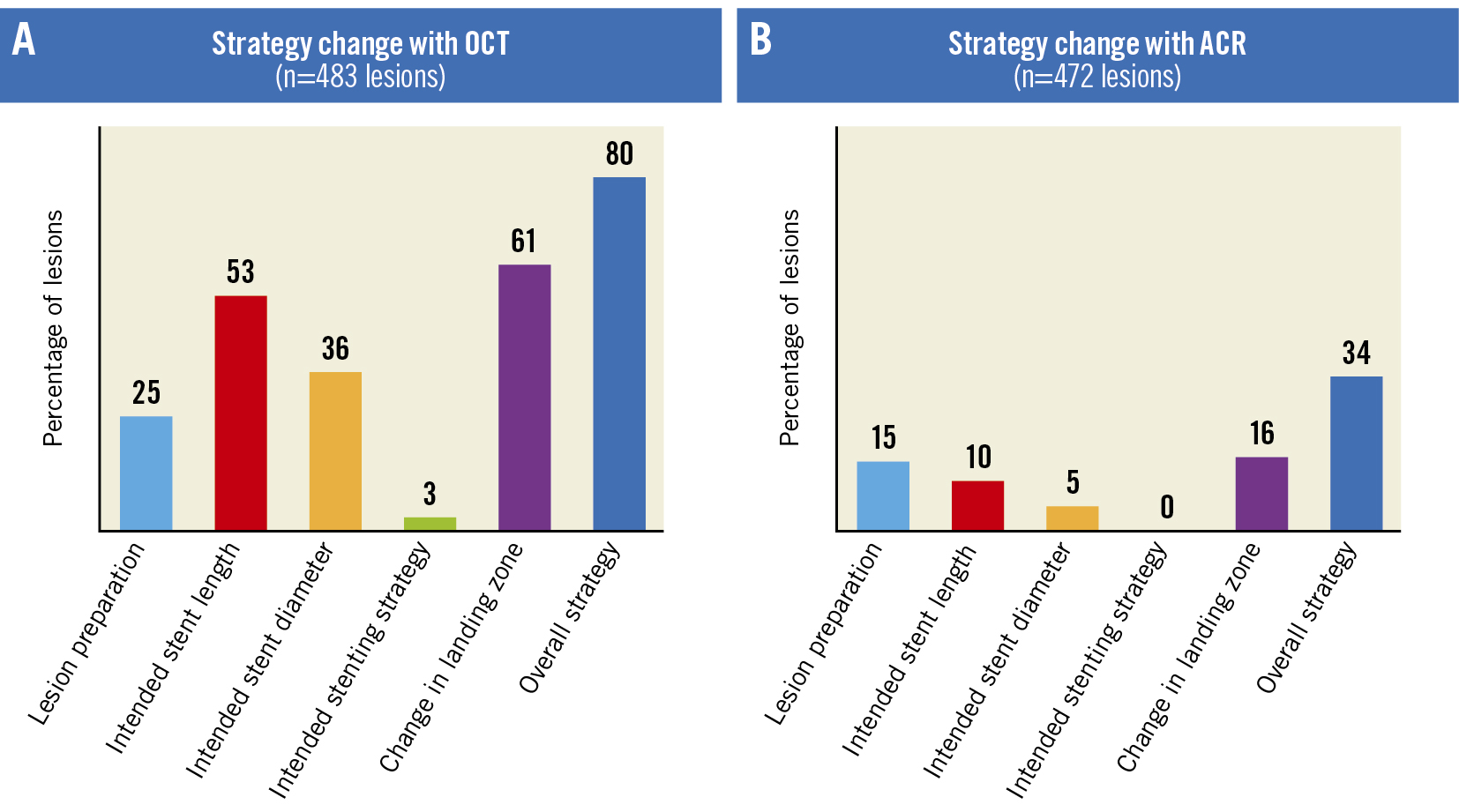
Figure 2. Strategy changes with OCT and ACR. A) Change in strategy with OCT (n=483 lesions). B) Change in strategy with ACR (n=472 lesions). ACR: angiographic coregistration; OCT: optical coherence tomography
Post-PCI strategy change
Postprocedural OCT demonstrated edge dissections (3%), underexpansion (15%), malapposition (14%), and tissue/thrombus prolapse (7%), which required additional interventions (ballooning/stenting) in 30% of lesions (Figure 3). The addition of ACR to OCT did not result in any further strategy change post-PCI. Thus, postprocedural ACR with OCT resulted in an overall strategy change in 30% of lesions.
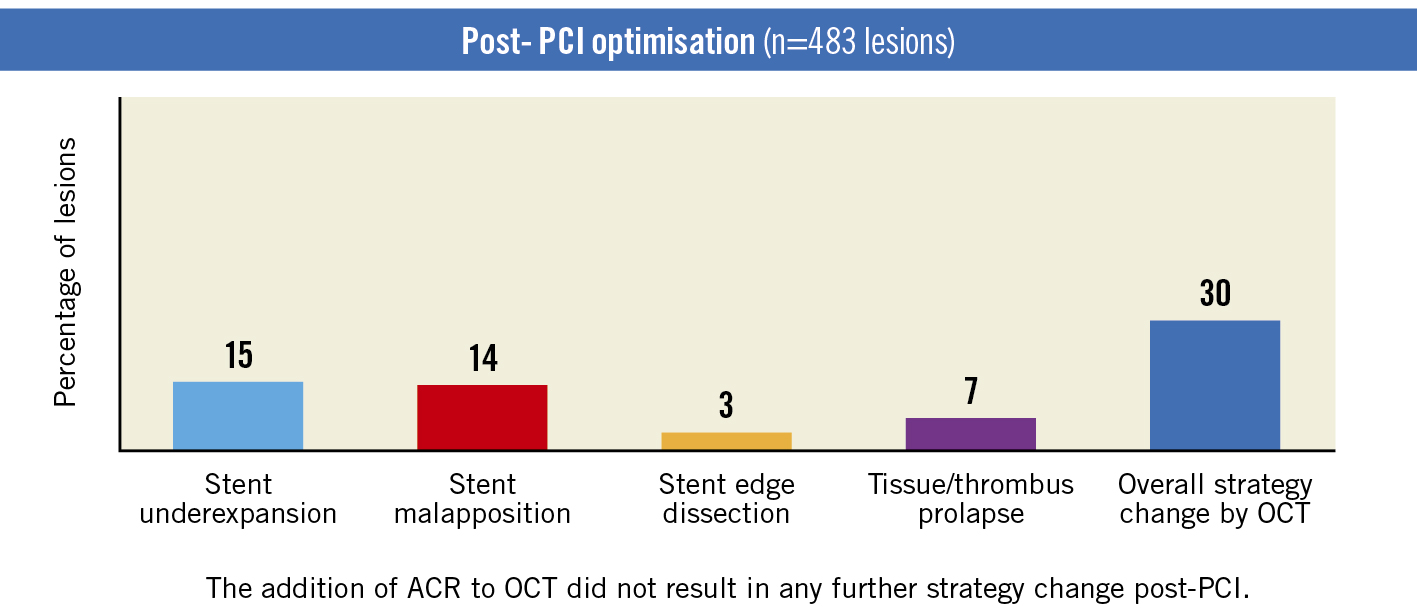
Figure 3. Post-PCI strategy change (OCT; n=483 lesions). ACR: angiographic coregistration; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Overall strategy change
Pre- and postprocedural ACR with OCT was associated with an overall strategy change in 89% of the lesions (Central illustration).
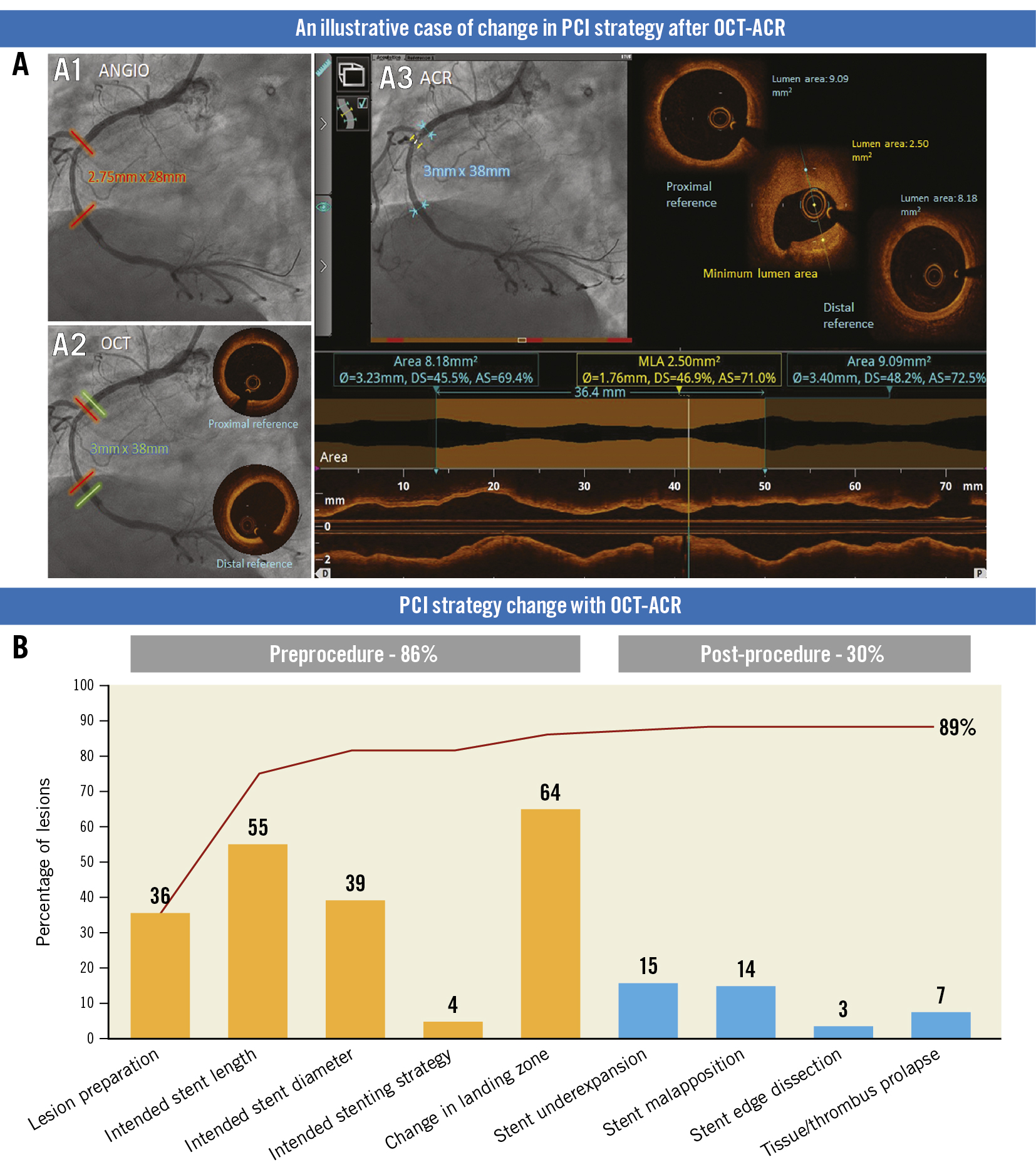
Central illustration. OCT-ACR guided decision-making during PCI. A) An illustrative case of change in PCI strategy after OCT-ACR. A1, A2, and A3 show stent length, diameter and landing zone based on angiogram, OCT and OCT-ACR, respectively. B) PCI strategy change with OCT-ACR. ACR: angiographic coregistration; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Secondary outcomes
Angiographic and procedural success was achieved in 100% of patients. One-year follow-up data for 470 patients were available. The incidence of MACE at 1 year was 0.85% (including cardiac death in two patients [0.4%], MI in one patient [0.2%], and TLR in one patient [0.2%]). The adverse events that resulted in hospitalisation are provided in Supplementary Table 1. The total amount of contrast used for the procedure was 210.0±106.6 mL, which included 41.6±20.1 mL for OCT and ACR. The time taken for OCT and ACR is summarised in Table 2.
Discussion
Detailed characterisation of the lesion morphology and accurate vessel sizing with OCT can improve procedural outcomes. While pre-PCI OCT can guide the procedural strategy, post-PCI OCT assists in detecting suboptimal stent deployment, which may not be detected by angiography9. ACR along with OCT can further improve PCI outcomes, and the feasibility of coregistration of OCT with angiography during PCI has been established in earlier studies78.
The OPTICO-Integration study was one of the first studies in the South East Asia region that evaluated OCT guidance with real-time ACR in a cohort of 50 patients and reported that OCT plus ACR improves PCI results. Notably, preprocedural OCT imaging altered the PCI strategy in 71% of lesions in the OPTICO-Integration study. Moreover, the use of ACR led to changes in PCI strategy in 40.7% of lesions10. The current multicentre, prospective iOPTICO study was performed in a similar real-world setting with a larger sample size (500 patients). Pre-PCI OCT was associated with a change in PCI strategy in 80% of lesions, and the addition of real-time ACR to OCT led to a strategy change in 34% of lesions. Optimisation was required in 30% of lesions when OCT was performed post-PCI.
The use of ACR facilitates the localisation of OCT morphology accurately on the angiogram monitor. This, in turn, enables precise identification of the landing zones when there are no angiographic markers, such as side branches, especially when the disease is diffuse, in which case landing zones with lipid-rich plaque and fibrous plaque appear similar on the angiogram. A previous study has shown a significant reduction in edge restenosis when appropriate landing zones were identified with OCT11. In particular, stent length and stent diameter can be more accurately assessed with OCT and ACR compared to CA. A significant difference was noted in the current study when the stent length and diameter values assessed with CA were compared with OCT and ACR. Accordingly, accurate identification of the landing zone following ACR in the current study led to a strategy change in over 50% of lesions, including a change in the stent length and stent diameter and further lesion preparation.
OCT after PCI also helps to identify markers of suboptimal stent deployment that are not apparent on the angiogram. In the CLI-OPCI study12, OCT helped detect edge dissection, lumen narrowing, stent malapposition, stent underexpansion and thrombi, which could not be detected in the angio group in 34.7% of cases. Similarly, in the ILUMIEN II trial13, edge dissection, malapposition and underexpansion were observed post-PCI, which led to further optimisation in 25% of patients. According to the ILUMIEN III: OPTIMIZE PCI study14, better resolution of OCT helped detect more stent malapposition and edge dissections, compared to IVUS or angiography. Similarly, the DOCTORS15 and OCTACS16 trials also reported a higher incidence of post-stent optimisation procedures, such as post-dilatation and additional stent implantations with OCT-guided PCI versus angiography-guided PCI. In the OPTICO-Integration study10, postprocedural OCT led to PCI optimisation in 52% of cases, which predominantly included stent malapposition and stent underexpansion. Similarly, in the present study, post-PCI optimisation was required in 30% of lesions, owing to stent malapposition, underexpansion, edge dissection and tissue prolapse.
In the OPTICO-Integration study10, postprocedural ACR did not have any further impact on the PCI strategy. Similarly, in the present study, the addition of ACR to OCT did not yield any additional changes in the post-PCI strategy. The presence of a stent with apposition and expansion indicators from the OCT pullback itself serves as a roadmap for the physician; hence, ACR did not likely influence post-PCI optimisation.
New insights from the LightLab initiative were presented recently. According to the outcomes reported, CA guidance was associated with inaccurate stent diameter assessment in 38% of the lesions (https://www.cardiovascular.abbott/content/dam/bss/divisionalsites/cv/pdf/reports/Abbott-LightLab-ePCR-Presentation.pdf. [Last accessed 04 March 2023]).
Lesion characteristics in the current study were similar to those reported in the LightLab initiative, with the majority of the lesions noted in the left anterior descending artery. The cumulative impact of OCT in the LightLab initative was a change in PCI strategy in 88% of lesions, which was similar to the strategy change observed in the present study. Further, while pre-PCI OCT led to a strategy change in 83% of lesions, post-PCI OCT resulted in a PCI strategy change in 31% of lesions in the LightLab study (https://www.cardiovascular.abbott/content/dam/bss/divisionalsites/cv/pdf/reports/Abbott-LightLab-ePCR-Presentation.pdf. [Last accessed 04 March 2023]). The corresponding figures in the present study were also similar (86% and 30%, respectively).
The average time taken in the present study for OCT imaging was 29 minutes, which was considerably lower compared to contemporary studies, such as the DOCTORS Study (56 mins)17 and the OPTICO-integration II Study (40 mins)18. The total amount of contrast used (210.0±106.6 mL) was comparable to amount used in the OPTICO-integration II Study (200 mL)18 and the DOCTORS Study (190 mL)17.
PCI optimisation following the use of OCT improves clinical outcomes in patients undergoing PCI1920. In the Pan-London PCI cohort study, OCT-guided PCI was associated with better procedural success rates and reduced in-hospital MACE, which translated into a reduction in mortality at 4.8-year follow-up19. Moreover, mortality was significantly lower in the CA plus OCT-guided group versus the CA group (hazard ratio [HR] 0.39, 95% confidence interval [CI]: 0.21-0.77; p=0.0008) and similar to the CA plus IVUS-guided group (HR 0.88, 95% CI: 0.61-1.38; p=0.43)19. The CLI-OPCI registry also observed an 8.3% reduction in the composite of cardiac death, MI, or repeat revascularisation at 1 year when OCT guidance was used for PCI20. In the present study, 100% procedural and angiographic success was achieved with no in-hospital MACE. Further, the procedural strategy with OCT- and ACR-guided PCI translated into better clinical outcomes at 12 months, with the overall incidence of MACE being <1%.
The outcomes related to the use of drug-eluting stents (DES) in the present study can be compared with those of two real-world studies, involving patients with complex lesions, conducted in India and the USA (XIENCE V [XV] India21 and XV USA22). The placement of DES in these two studies was based on findings noted on CA. The XV India and XV USA studies reported a 1-year MACE rate of 5% and 6.9%, respectively, following DES implantation. Notably, the rates of death and TLR were significantly lower in the present study compared to the XV USA study (p=0.0401 and p<0.0001, respectively), and the incidence of MI was significantly lower in the present study compared to both the XV USA and XV India studies (p=0.0421 and p<0.0001, respectively) (Figure 4)2122. The low incidence of MACE seen in the current study could be attributed to the use of OCT and ACR, which resulted in stent optimisation and, consequently, better 1-year outcomes.
Notably, the use of ACR with OCT is currently not limited to clinical research and is being increasingly preferred as an option in the busy catheterisation laboratories (despite the additional time required) owing to improved MACE rates. The current study reflects the actual benefits noted in a real-world scenario, especially in patients with complex lesions. The improved outcomes noted in the study may be attributed to better characterisation of the lesion morphology, accurate vessel sizing, and optimal strategy change following pre- and postprocedural OCT with ACR (including change in the stent length and stent diameter and additional lesion preparation).
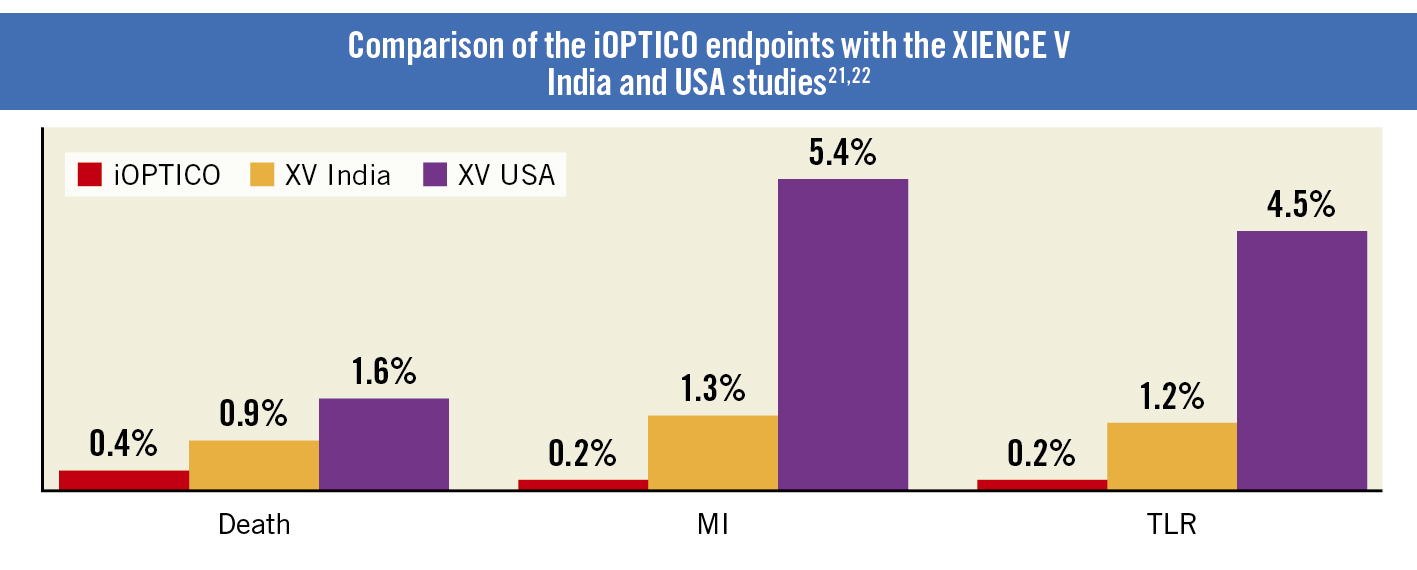
Figure 4. Comparison of the iOPTICO endpoints with the XV India and XV USA studies. MI: myocardial infarction; TLR: target lesion revascularisation
Limitations
The present study is limited by its observational and non-randomised design. There is a possibility of “operator bias” as the operators were not blinded to the study. Nevertheless, the iOPTICO study reiterates the findings reported in other studies, albeit in a real-world scenario with a larger sample size. OCT with ACR was associated with a higher percentage of change in PCI strategy compared to earlier studies, suggesting a potential improvement in the technology. Notably, the overall incidence of MACE was <1%, which supports the belief that better procedural strategy with OCT- and ACR-guided PCI translates into improved clinical outcomes.
Conclusions
The study reflects the real-time benefits of ACR with OCT in patients undergoing PCI. The use of OCT resulted in a significant change in the overall preprocedural strategy, while real-time ACR had an additional significant impact on the treatment strategy with good long-term outcomes at 1 year after the index PCI.
Impact on daily practice
Intracoronary imaging with OCT ushers in a precision era of PCI planning and optimisation by providing enhanced visualisation and high-resolution imaging of the coronary artery. The current iOPTICO study hypothesised that the utility of OCT along with ACR will considerably impact the clinician’s decision-making and overall procedural strategy compared to angiography. Preprocedural OCT resulted in a change in PCI strategy in 80% of lesions, with an additional change in treatment strategy in 34% of lesions when ACR was used. Post-PCI, OCT helped identify a need for further PCI optimisation in 30% of lesions. The impact of OCT imaging on decision-making is pronounced, being additionally and incrementally beneficial when performed preprocedurally with ACR. Post-PCI, identifying the need for additional intervention with OCT helps in optimising stent implantation. Taken together, this results in better clinical outcomes at 1 year; hence, OCT with ACR may play an important role in decision-making during coronary interventions and may translate into best clinical practices, especially in complex coronary lesions.
Acknowledgements
The authors would like to thank all the study staff (co-investigators and research coordinators) for their support in executing the study, St. Jude Medical India Pvt. Ltd. (now Abbott) for the research grant, and BioQuest Solutions Pvt. Ltd. for supporting the execution of the study.
Funding
The study was funded by St. Jude Medical India Pvt. Ltd. (now Abbott). The funding agency had no role in the study design, execution, analysis, or interpretation of results.
Conflict of interest statement
The authors have no conflicts of interest to declare.

