An 84-year-old female with a history of aortic valve replacement, implanted with a 19 mm Mosaic (Medtronic) valve, presented with worsening dyspnoea. Transthoracic echocardiography revealed severe stenosis of the prosthetic aortic valve. Because of the high surgical risk, the Heart Team decided on valve-in-valve transcatheter aortic valve implantation (ViV-TAVI) with an Evolut PRO 23 mm (Medtronic) valve. As the patient’s anatomy put her at high risk for coronary occlusions, our strategy was to deploy the transcatheter heart valve (THV) at a lower position than recommended in order to prevent coronary occlusion (Figure 1A–Figure 1C). After valve deployment, coronary occlusion was avoided; however, a complete left bundle branch block was noted (Figure 1D, Figure 1E). On the fourth day after the procedure, the patient developed a complete atrioventricular (AV) block requiring permanent pacemaker implantation (PPI) (Figure 1F).
Although the incidence of PPI in ViV-TAVI is low1, this patient’s membranous septum length was 3.4 mm, and the risk of conduction disturbances had to be taken into consideration (Figure 1G)2. To verify the mechanism, an ex vivo simulation of the ViV-TAVR was conducted under the same conditions, altering the implantation position by 3 mm (Figure 1H–Figure 1J). This experiment proved that when the THV is implanted lower than the recommended position, the THV inflow expands significantly without making a significant difference in THV expansion at the eyelet level of the Mosaic valve, which suggests that a deep implantation has minimal protective impact on avoiding coronary occlusion (Figure 1K). Our case highlights the importance of deployment in the recommended position for successful ViV-TAVI.
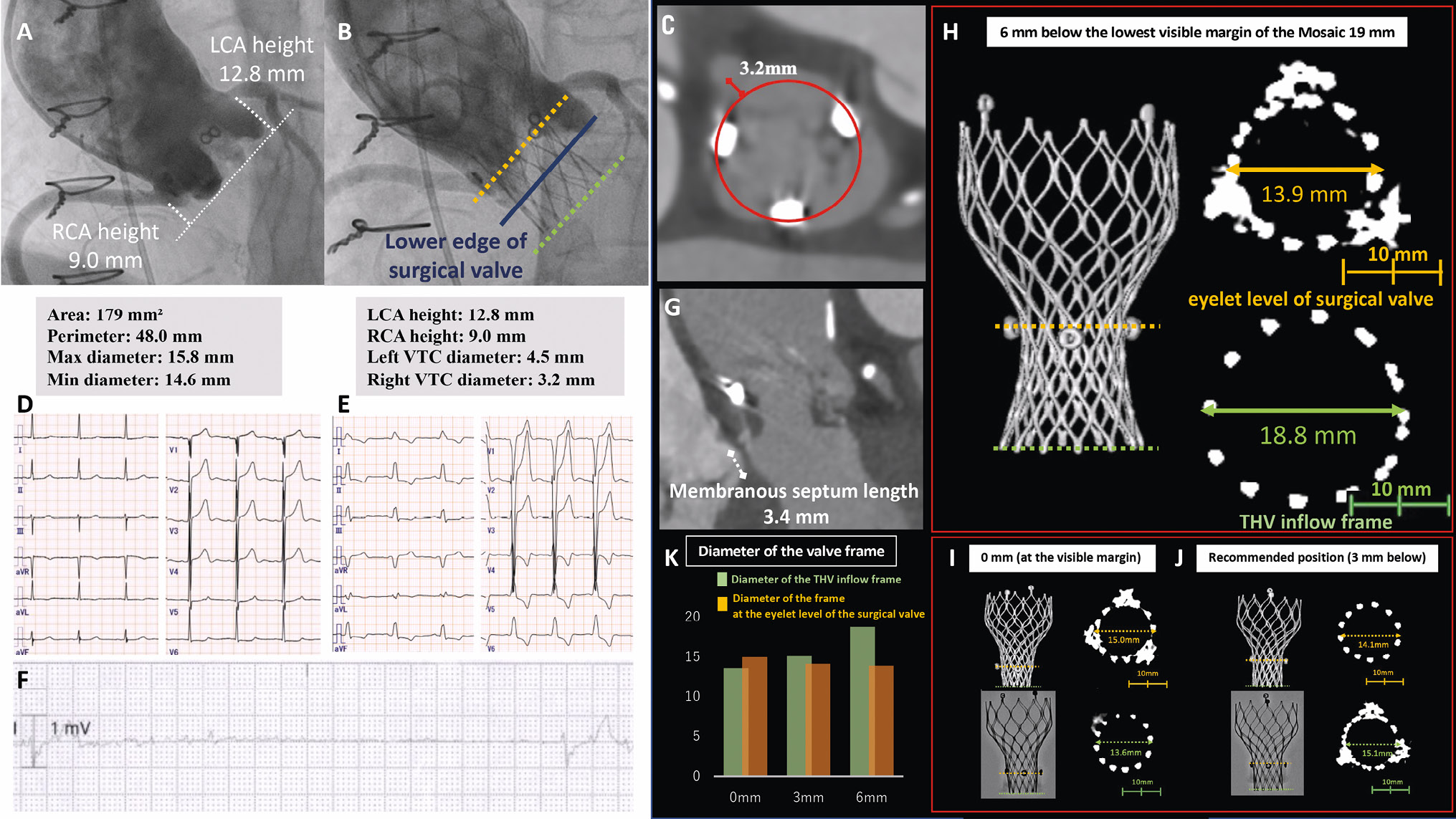
Figure 1. Procedural details, clinical events, and analysis of impact on implantation depth in an ex vivo experiment. A, B) Pre- and postprocedural aortography with measurements of the computed tomography (CT) scan parameters. C) CT scan showing the short virtual transcatheter valve-to-coronary ostia (VTC) distance. D, E) Pre- and postoperative electrocardiograms. F) Electrocardiographic monitoring when the block appears. G) CT scan showing the membranous septal length. H-J) Angiographic and CT scan appearance in the ex vivo experiment using a Mosaic 19 mm bioprosthesis and an Evolut PRO 23 mm at the implantation positions of 0 mm (at the visible margin), 3 mm below the visible margin (the recommended position), and 6 mm below the visible margin. K) Summary of the diameter of the valve frame. LCA: left coronary artery; RCA: right coronary artery; THV: transcatheter heart valve
Conflict of interest statement
K. Takagi has received lecture fees from Abbott Medical Japan Co, Edwards Lifesciences, and Daiichi Sankyo. The other authors have no conflicts of interest to declare.

