Currently, percutaneous coronary intervention (PCI) with drug-eluting stents (DES) is the most prevalent revascularisation treatment approach for coronary artery disease (CAD)12. Modern DES technologies promise advantages of early re-endothelialisation and decreased rates of restenosis2345. Moreover, DES with biodegradable polymers have the potential to mitigate the persistent inflammatory reactions induced by permanent polymers4. Thus, new-generation biodegradable polymer-coated DES have a positive impact, especially in patients with anatomically complex lesions and among patients associated with increased rates of major adverse cardiac events (MACE) after PCI3. Supraflex Cruz (Sahajanand Medical Technologies Ltd.) is one such new-generation biodegradable polymer-coated sirolimus-eluting stent (SES) with long dual Z connectors (LDZ links) from “valley-to-valley” between the struts, which enhance deliverability and increase the flexibility of the stent, and a redesigned proximal shaft to allow better pushability4. Nevertheless, real-world evidence concerning Supraflex Cruz SES is limited to specific populations, prompting the need for evidence across various ethnicities and geographical locations. Therefore, the S-FLEX Russia registry was designed to evaluate the clinical safety and performance of the Supraflex Cruz SES in a real-world, all-comers population with CAD, including high-risk subgroups in Russia.
Methods
Study design and population
The S-FLEX Russia registry was a prospective, multicentre, single-arm, observational registry which included 522 patients who underwent PCI with the Supraflex Cruz SES between August 2021 and February 2022, at five tertiary care centres in Russia. The study protocol was approved by the Institutional Ethics Committee of each respective centre, and the study was conducted in accordance with good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Patients (aged ≥18 years) enrolled in this registry had symptomatic CAD and a clinical indication for PCI, and they were willing to participate in all follow-up assessments as per the protocol. Patients were excluded if they had cardiogenic shock, planned surgery within 12 months of PCI, known hypersensitivity or contraindication to aspirin, heparin, clopidogrel, or any other antiplatelets/anticoagulants required for PCI, sirolimus, cobalt chromium, or contrast media; additionally, pregnant female patients and patients who were participating in a clinical study of another drug or medical device were also excluded.
Study device
Supraflex Cruz is the new-generation Supraflex stent, constructed on an L-605 cobalt-chromium alloy platform. It has an open-cell design and dual valley-to-valley connection between strut rings with alternate LDZ links. The stent is uniformly coated with sirolimus (1.4 μg/mm2) blended with biodegradable polymers (4-6 μm). It has a multilayer drug-polymer coating which consists of a combination of hydrophobic (poly L-lactic acid [PLLA] and poly L-lactide-co-ε-caprolactone [PLCL]) polymers blended with sirolimus in the middle and innermost layer, and the outer coating is a drug-free hydrophilic polymer (polyvinyl pyrrolidone [PVP]). Nearly 80% of the drug is released within 1 month of stent implantation, and the remaining drug is gradually released over the subsequent 3 months. The polymers undergo gradual degradation and are excreted as biologically inert molecules within 10 to 12 months after implantation.
Interventional procedure and follow-up
Lesion severity was evaluated through visual estimation following invasive coronary angiography, and subsequent revascularisation and post-procedure management were conducted in accordance with routine practice. Dual antiplatelet therapy (DAPT) was recommended for at least 12 months in patients with acute coronary syndrome (ACS) and 6 months in patients with stable CAD. All patients were followed up via clinic visit or telephone communication at 30 days and 12 months post-PCI.
Study endpoints and definitions
The primary endpoint was the rate of target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (TVMI) and clinically driven target lesion revascularisation (CD-TLR) at 12 months6. The secondary safety endpoints involved overall stent thrombosis (definite/probable), all-cause death (cardiac, vascular, and non-cardiovascular), and any myocardial infarction (MI) at 12 months6. Cardiac death was defined as death due to any cardiac mechanisms (fatal arrhythmia, sudden death, MI, or low cardiac output heart failure), any unwitnessed death, death of unknown cause, or procedure- related death67. TVMI was adjudged as per the 4th universal definition of myocardial infarction (UDMI). It was defined as target vessel Q-wave or non-Q-wave MI with evidence of myocardial ischaemia in the vascular territory of the previously treated target vessel8. CD-TLR was defined as any repeat revascularisation to treat the stented segment (including 5 mm proximal or 5 mm distal to the stent margins) demonstrated as ≥50% diameter stenosis on quantitative coronary angiography at the target lesion7. Target vessel revascularisation (TVR) was defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel including the target lesion7. Stent thrombosis was defined as per the Academic Research Consortium 2 (ARC-2) definition6.
Device success was defined as successful delivery and deployment of the study device (Supraflex Cruz SES) at the designated target lesion, along with successful retrieval of the stent delivery system and achievement of final residual stenosis of <30% on visual estimation. Procedural success was defined as accomplishment of device success without any adverse cardiac event occurring during the index hospital stay.
Statistical analysis
Continuous variables are expressed as mean±standard deviation, whereas categorical variables are represented as frequency (percentage). Cumulative rates of events up to 12 months were assessed using the Kaplan-Meier method. In addition, a subgroup analysis was undertaken, wherein clinical outcomes were assessed for patients with diabetes mellitus (those who were either known to be diabetic and on pharmacological treatment or who had documented glycated haemoglobin >7% even if they were not on pharmacological treatment), bifurcation coronary lesions, type B2/C lesions (defined according to ACC/AHA lesion classification)9, and long coronary lesions (target lesion length of >20 mm). The data were analysed using R software, version 4.3.1 (R Foundation for Statistical Computing).
Results
A total of 522 patients were included in the study. The mean age of the patients was 64.26±9.46 years, and 70.7% were male. Of 24.1% (n=126) patients with diabetes mellitus, 18.1% patients were insulin dependent. A total of 43.3% (226/522) patients presented with ACS, and multivessel disease was present in 198 (37.9%) patients. Severe left ventricular dysfunction (ejection fraction ≤35%) was reported in 14 (2.7%) patients. The baseline clinical characteristics of the entire cohort and prespecified subgroups are provided in Table 1.
A total of 739 Supraflex Cruz SES were implanted to treat 621 lesions. The detailed lesion and procedural characteristics are outlined in Table 2 and Table 3, respectively. Moderate to heavy calcification was present in 130 (20.9%) lesions. Bifurcation was present in 74/602 (12.3%) lesions, and chronic total occlusion (≥3-month-old occlusion) was reported in 48 (7.7%) lesions. Overall, the mean reference vessel diameter was 3.17±0.49 mm, which represented a mean diameter stenosis of 84.77±11.61%. The mean stent length was 25.51±9.14 mm, and the mean stent diameter was 3.18±0.49 mm. Device success was achieved in 99.4% of lesions (617/621), with four cases of device failure (one case of stent dislodgment, one case of delivery system crossing failure, and two cases due to residual stenosis of >20%). Additionally, procedural success was achieved in 98.9% of patients (516/522), with six cases of procedural failure – four related to device failure and two due to TVMI. The details of antiplatelet medication prescribed at discharge and its adherence at discharge, and 30-day and 12-month follow-up are listed in Table 4.
Clinical outcomes at 30-day and 12-month follow-up are provided in Table 5. At 12-month follow-up, the cumulative incidence of the primary endpoint (TLF) was 3.1%, comprising 1.5% (n=8) cardiac death, 1.1% (n=6) TVMI, and 0.4% (n=2) CD-TLR. At 12 months, 8 (1.5%) patients underwent TVR. Definite/probable stent thrombosis was observed in only one patient (0.2%) at 12 months. In the prespecified subgroups, the incidence of TLF at 12 months was 5.6% for diabetes mellitus, 8.5% for bifurcations, 2.8% for type B2/C lesions, and 1.9% for long lesions (>20 mm). The time-to-event Kaplan-Meier curves for TLF up to 12-month follow-up are illustrated in Figure 1 (overall population) and Figure 2 (all four subgroups).
Table 1. Baseline clinical characteristics.
| Characteristics | Total (n=522) |
Diabetes (n=126) |
Bifurcation (n=71) |
B2/C lesion (n=429) |
Long lesion (>20 mm) (n=268) |
|---|---|---|---|---|---|
| Age, years | 64.26±9.46 | 65.99±8.89 | 62.85±8.88 | 64.35±9.34 | 64.35±9.73 |
| Sex | |||||
| Male | 369 (70.7) | 69 (54.8) | 54 (76.1) | 299 (69.7) | 187 (69.8) |
| Female | 153 (29.3) | 57 (45.2) | 17 (23.9) | 130 (30.3) | 81 (30.2) |
| Height, cm | 168.94±9.98 | 166.64±10.05 | 168.65±8.72 | 168.69±10.29 | 168.47±10.64 |
| Weight, kg | 84.88±18.11 | 87.25±21.48 | 84.80±16.93 | 84.73±18.55 | 85.25±18.54 |
| Body mass index, kg/m2 | 29.94±8.86 | 31.34±6.98 | 29.81±5.78 | 30.02±9.47 | 30.47±11.22 |
| Coexisting illness and addiction | |||||
| Diabetes mellitus | 126 (24.1) | 126 (100) | 17 (23.9) | 108 (25.2) | 67 (25.0) |
| Requiring insulin | 21/116 (18.1) | 21/116 (18.1) | 6/16 (37.5) | 18/99 (18.2) | 11/61 (18.0) |
| Not requiring insulin | 95/116 (81.9) | 95/116 (81.9) | 10/16 (62.5) | 81/99 (81.8) | 50/61 (82.0) |
| Hypertension | 491 (94.1) | 123 (97.6) | 69 (97.2) | 404 (94.2) | 253 (94.4) |
| Renal insufficiency | 22 (4.2) | 5 (4.0) | 3 (4.2) | 17 (4.0) | 11 (4.1) |
| Family history of CAD | 112 (21.5) | 30 (23.8) | 16 (22.5) | 96 (22.4) | 66 (24.6) |
| Peripheral vascular disease | 166 (31.8) | 40 (31.7) | 19 (26.8) | 140 (32.6) | 100 (37.3) |
| Smoker | 137 (26.2) | 26 (20.6) | 17 (23.9) | 113 (26.3) | 67 (25.0) |
| Hypercholesterolaemia | 276 (52.9) | 68 (54.0) | 35 (49.3) | 235 (54.8) | 149 (55.6) |
| Congestive heart failure | 168 (32.2) | 46 (36.5) | 25 (35.2) | 153 (35.7) | 103 (38.4) |
| Transient ischaemic attack | 8 (1.5) | 1 (0.8) | 2 (2.8) | 6 (1.4) | 5 (1.9) |
| Previous stroke | 24 (4.6) | 7 (5.6) | 3 (4.2) | 20 (4.7) | 16 (6.0) |
| Previous MI | 179 (34.3) | 53 (42.1) | 24 (33.8) | 154 (35.9) | 103 (38.4) |
| Previous PCI | 176 (33.7) | 57 (45.2) | 27 (38.0) | 150 (35.0) | 93 (34.7) |
| Previous CABG | 11 (2.1) | 4 (3.2) | 3 (4.2) | 10 (2.3) | 9 (3.4) |
| Clinical presentation | |||||
| NSTEMI | 26 (5.0) | 6 (4.8) | 6 (8.5) | 21 (4.9) | 12 (4.5) |
| STEMI | 88 (16.9) | 14 (11.1) | 13 (18.3) | 73 (17.0) | 40 (14.9) |
| Unstable angina | 112 (21.5) | 29 (23.0) | 8 (11.3) | 86 (20.0) | 46 (17.2) |
| Silent ischaemia | 31 (5.9) | 10 (7.9) | 5 (7.0) | 30 (7.0) | 22 (8.2) |
| Stable angina | 265 (50.8) | 67 (53.2) | 39 (54.9) | 219 (51.0) | 148 (55.2) |
| Extent of CAD | |||||
| Single-vessel disease | 324 (62.1) | 80 (63.5) | 43 (60.6) | 265 (61.8) | 150 (56.0) |
| Multivessel disease | 198 (37.9) | 46 (36.5) | 28 (39.4) | 164 (38.2) | 118 (44.0) |
| Ejection fraction | |||||
| Good (≥60%) | 171 (32.8) | 41 (32.5) | 29 (40.8) | 122 (28.4) | 66 (24.6) |
| Moderate (>35% to <60%) | 297 (56.9) | 76 (60.3) | 34 (47.9) | 258 (60.1) | 174 (64.9) |
| Severe (≤35%) | 14 (2.7) | 2 (1.6) | 4 (5.6) | 11 (2.6) | 6 (2.2) |
| Unknown | 40 (7.7) | 7 (5.6) | 4 (5.6) | 38 (8.9) | 22 (8.2) |
| Data are mean±SD, n (%), or n/N (%) in case of missing data. CABG: coronary artery bypass grafting; CAD: coronary artery disease; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction | |||||
Table 2. Target vessel and lesion characteristics.
| Characteristics | Total (621 lesions) |
Diabetes (156 lesions) |
Bifurcation (74 lesions) |
B2/C lesion (488 lesions) |
Long lesion (>20 mm) (328 lesions) |
|---|---|---|---|---|---|
| Target vessel | |||||
| Left anterior descending artery | 244 (39.3) | 60 (38.5) | 38 (51.4) | 195 (40.0) | 124 (37.8) |
| Right coronary artery | 207 (33.3) | 55 (35.3) | 21 (28.4) | 170 (34.8) | 128 (39.0) |
| Left circumflex artery | 157 (25.3) | 39 (25.0) | 8 (10.8) | 113 (23.2) | 68 (20.7) |
| Left main artery | 12 (1.9) | 2 (1.3) | 7 (9.5) | 9 (1.8) | 8 (2.4) |
| Saphenous vein graft | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Lesion characteristics | |||||
| AHA/ACC lesion classification | |||||
| Type A | 26 (4.2) | 4 (2.6) | 1 (1.4) | – | 2 (0.6) |
| Type B1 | 107 (17.2) | 30 (19.2) | 5 (6.8) | – | 14 (4.3) |
| Type B2 | 161 (25.9) | 41 (26.3) | 30 (40.5) | 161 (33.0) | 19 (5.8) |
| Type C | 327 (52.7) | 81 (51.9) | 38 (51.4) | 327 (67.0) | 293 (89.3) |
| Length | |||||
| Discrete (<10 mm) | 81 (13.0) | 23 (14.7) | 14 (18.9) | 27 (5.5) | 10 (3.0) |
| Tubular (10-20 mm) | 250 (40.3) | 64 (41.0) | 29 (39.2) | 171 (35.0) | 28 (8.5) |
| Diffuse (>20 mm) | 290 (46.7) | 69 (44.2) | 31 (41.9) | 290 (59.4) | 290 (88.4) |
| Eccentricity | |||||
| Concentric | 512 (82.4) | 120 (76.9) | 63 (85.1) | 382 (78.3) | 266 (81.1) |
| Eccentric | 109 (17.6) | 36 (23.1) | 11 (14.9) | 106 (21.7) | 62 (18.9) |
| Accessibility | |||||
| Readily accessible tortuosity of proximal segment | 564 (90.8) | 141 (90.4) | 65 (87.8) | 432 (88.5) | 292 (89.0) |
| Moderate tortuosity of proximal segment | 56 (9.0) | 15 (9.6) | 9 (12.2) | 55 (11.3) | 35 (10.7) |
| Excessive | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.3) |
| Lesion angulation | |||||
| None (<45º) | 538 (86.6) | 131 (84.0) | 61 (82.4) | 406 (83.2) | 268 (81.7) |
| Moderate (≥45º and <90º) | 78 (12.6) | 22 (14.1) | 12 (16.2) | 77 (15.8) | 58 (17.7) |
| Location in severe bend point (≥90º) | 5 (0.8) | 3 (1.9) | 1 (1.4) | 5 (1.0) | 2 (0.6) |
| Lesion contour | |||||
| Smooth | 488 (78.6) | 122 (78.2) | 55 (74.3) | 359 (73.6) | 259 (79.0) |
| Irregular | 133 (21.4) | 34 (21.8) | 19 (25.7) | 129 (26.4) | 69 (21.0) |
| Bifurcation or side branch lesions | |||||
| No major branch involvement | 532 (85.7) | 134 (85.9) | 2 (2.7) | 404 (82.8) | 279 (85.1) |
| Bifurcation lesions requiring double guidewire | 81 (13.0) | 19 (12.2) | 71 (95.9) | 76 (15.6) | 42 (12.8) |
| Inability to protect major side branches | 8 (1.3) | 3 (1.9) | 1 (1.4) | 8 (1.6) | 7 (2.1) |
| Degenerated bypass grafts with unstable lesions | 9/445 (2.0) | 4/111 (3.6) | 0 (0) | 9/330 (2.7) | 1/213 (0.5) |
| Lesion complexity | |||||
| Lesion requiring overlapping stents | 63 (10.1) | 17 (10.9) | 11 (14.9) | 57 (11.7) | 57 (17.4) |
| Restenotic lesion | 28 (4.5) | 12 (7.7) | 4 (5.4) | 25 (5.1) | 21 (6.4) |
| Bifurcation | 74/602 (12.3) | 17/154 (11.0) | 74 (100) | 68/474 (14.3) | 38/318 (11.9) |
| Moderate to heavy calcification | 130 (20.9) | 39 (25.0) | 18 (24.4) | 124 (25.4) | 78 (23.8) |
| Ostial (within 3 mm of vessel origin) | 66 (10.6) | 20 (12.8) | 18 (24.3) | 60 (12.3) | 38 (11.6) |
| Thrombus | 59 (9.5) | 10 (6.4) | 10 (13.5) | 59 (12.1) | 28 (8.5) |
| Total occlusion (<3 months) | 45 (7.2) | 9 (5.8) | 4 (5.4) | 43 (8.8) | 22 (6.7) |
| Chronic total occlusion (≥3 months) | 48 (7.7) | 13 (8.3) | 12 (16.2) | 48 (9.8) | 40 (12.2) |
| Data are n (%), or n/N (%) in case of missing data. | |||||
Table 3. Procedural characteristics.
| Characteristics | Total (621 lesions) |
Diabetes (156 lesions) |
Bifurcation (74 lesions) |
B2/C lesion (488 lesions) |
Long lesion (>20 mm) (328 lesions) |
| Reference vessel diameter, mm | 3.17±0.49 | 3.14±0.47 | 3.10±0.45 | 3.19±0.47 | 3.21±0.48 |
| Diameter stenosis, % | 84.77±11.61 | 83.99±11.26 | 85.05±14.61 | 85.39±12.07 | 85.49±11.95 |
| Predilatation | 444 (71.5) | 118 (75.6) | 59 (79.7) | 361 (74.0) | 261 (79.6) |
| Maximum inflation pressure per stent, atm | 12.38±3.00 (337/621) | 12.06±2.87 (88/156) | 12.73±3.00 (45/74) | 12.72±3.07 (261/488) | 12.88±2.62 |
| (178/328) | |||||
| Post-dilatation | 227 (36.6) | 52 (33.3) | 46 (62.2) | 191 (39.1) | 140 (42.7) |
| Maximum inflation pressure post-stenting, atm | 17.12±3.40 (211/621) | 16.81±3.43 (48/156) | 15.56±3.42 | 17.29±3.50 | 17.54±3.34 |
| (41/74) | (176/488) | (126/328) | |||
| Stents | n=739 | n=183 | n=96 | n=601 | n=434 |
| No. of stents per patient, mm | 1.42±0.72 | 1.45±0.68 | 1.35±0.51 | 1.40±0.72 | 1.62±0.82 |
| No. of stents per lesion, mm | 1.30±0.59 | 1.32±0.57 | 1.33±0.50 | 1.32±0.62 | 1.46±0.69 |
| Total stent length per patient, mm | 36.11±21.70 | 35.40±19.59 | 34.65±17.04 | 37.63±22.06 | 46.61±23.33 |
| Total stent length per vessel, mm | 33.19±19.00 | 32.09±16.44 | 34.17±16.69 | 35.56±19.47 | 42.06±20.63 |
| Mean stent length, mm | 25.51±9.14 | 24.37±8.52 | 25.63±8.32 | 26.86±9.11 | 28.78±9.30 |
| Mean stent diameter, mm | 3.18±0.49 | 3.14±0.46 | 3.08±0.43 | 3.18±0.48 | 3.18±0.49 |
| Device success | 617/621 (99.4) | 155/156 (99.4) | 73/74 (98.6) | 484/488 (99.2) | 326/328 (99.4) |
| Procedural success | 516/522 (98.9) | 125/126 (99.2) | 69/71 (97.2) | 424/429 (98.8) | 266/268 (99.3) |
| Data are mean±standard deviation, n (%), or n/N (%) in case of missing data. | |||||
Table 4. Details of antiplatelet medication at discharge, and 30-day and 12-month follow-up.
| Characteristics | Total (n=522) |
Diabetes (n=126) |
Bifurcation (n=71) |
B2/C lesion (n=429) |
Long lesion (>20 mm) (n=268) |
|---|---|---|---|---|---|
| Medication at discharge | |||||
| Aspirin | 503 (96.4) | 116 (92.1) | 68 (95.8) | 411 (95.8) | 257 (95.9) |
| Antiplatelet therapy | 507 (97.1) | 122 (96.8) | 68 (95.8) | 417 (97.2) | 262 (97.8) |
| Clopidogrel | 388 (74.3) | 103 (81.7) | 57 (80.3) | 315 (73.4) | 206 (76.9) |
| Prasugrel | 4 (0.8) | 0 (0) | 2 (2.8) | 4 (0.9) | 3 (1.1) |
| Ticagrelor | 115 (22.0) | 19 (15.1) | 9 (12.7) | 98 (22.8) | 53 (19.8) |
| Aspirin+thienopyridine | 498 (95.4) | 116 (92.1) | 67 (94.4) | 408 (95.1) | 256 (95.5) |
| Oral anticoagulant | 86 (16.5) | 26 (20.6) | 14 (19.7) | 67 (15.6) | 39 (14.6) |
| Medication at 30-day follow-up | |||||
| Aspirin | 486 (93.1) | 115 (91.3) | 64 (90.1) | 399 (93.0) | 247 (92.2) |
| Antiplatelet therapy | 506 (96.9) | 125 (99.2) | 67 (94.4) | 418 (97.4) | 262 (97.8) |
| Clopidogrel | 396 (75.9) | 106 (84.1) | 55 (77.5) | 324 (75.5) | 214 (79.9) |
| Prasugrel | 4 (0.8) | 1 (0.8) | 2 (2.8) | 4 (0.9) | 2 (0.7) |
| Ticagrelor | 106 (20.3) | 18 (14.3) | 10 (14.1) | 90 (21.0) | 46 (17.2) |
| Aspirin+thienopyridine | 483 (92.5) | 114 (90.5) | 63 (88.7) | 398 (92.8) | 247 (92.2) |
| Medication at 12-month follow-up | |||||
| Aspirin | 472 (90.4) | 113 (89.7) | 62 (87.3) | 384 (89.5) | 244 (91.0) |
| Antiplatelet therapy | 486 (93.1) | 120 (95.2) | 65 (91.5) | 398 (92.8) | 252 (94.0) |
| Clopidogrel | 377 (72.2) | 100 (79.4) | 53 (74.6) | 306 (71.3) | 202 (75.4) |
| Prasugrel | 3 (0.6) | 0 (0) | 2 (2.8) | 3 (0.7) | 2 (0.7) |
| Ticagrelor | 106 (20.3) | 20 (15.9) | 10 (14.1) | 89 (20.7) | 48 (17.9) |
| Aspirin+thienopyridine | 462 (88.5) | 110 (87.3) | 60 (84.5) | 376 (87.6) | 239 (89.2) |
| Data are n (%). | |||||
Table 5. Clinical outcomes at 30-day and 12-month follow-up.
| Clinical outcomes | At 30-day follow-up | At 12-month follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=522) |
Diabetes (n=126) |
Bifurcation (n=71) |
B2/C lesion (n=429) |
Long lesion (>20 mm) (n=268) |
Total (n=522) |
Diabetes (n=126) |
Bifurcation (n=71) |
B2/C lesion (n=429) |
Long lesion (>20 mm) (n=268) |
|
| TLF | 4 (0.8) | 1 (0.8) | 2 (2.8) | 2 (0.5) | 0 (0) | 16 (3.1) | 7 (5.6) | 6 (8.5) | 12 (2.8) | 5 (1.9) |
| Target vessel failure | 4 (0.8) | 1 (0.8) | 2 (2.8) | 2 (0.5) | 0 (0) | 17 (3.3) | 8 (6.3) | 6 (8.5) | 13 (3.0) | 5 (1.9) |
| Death from any cause | 3 (0.6) | 0 (0) | 1 (1.4) | 2 (0.5) | 1 (0.4) | 17 (3.3) | 4 (3.2) | 5 (7.0) | 13 (3.0) | 6 (2.2) |
| Cardiac death | 1 (0.2) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 8 (1.5) | 3 (2.4) | 4 (5.6) | 6 (1.4) | 3 (1.1) |
| Non-cardiac death | 2 (0.4) | 0 (0) | 0 (0) | 2 (0.5) | 1 (0.4) | 9 (1.8) | 1 (0.8) | 1 (1.4) | 7 (1.6) | 3 (1.1) |
| All MI | 4 (0.8) | 1 (0.8) | 2 (2.8) | 3 (0.7) | 0 (0) | 8 (1.5) | 4 (3.2) | 3 (4.2) | 7 (1.6) | 3 (1.1) |
| TVMI | 3 (0.6) | 1 (0.8) | 1 (1.4) | 2 (0.5) | 0 (0) | 6 (1.1) | 3 (2.4) | 2 (2.8) | 5 (1.2) | 2 (0.7) |
| Non-TVMI | 1 (0.2) | 0 (0) | 1 (1.4) | 1 (0.2) | 0 (0) | 2 (0.4) | 1 (0.8) | 1 (1.4) | 2 (0.5) | 1 (0.4) |
| TLR | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 5 (1.0) | 1 (0.8) | 1 (1.4) | 4 (0.9) | 1 (0.4) |
| CD-TLR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.4) | 1 (0.8) | 0 (0) | 1 (0.2) | 0 (0) |
| Non-CD-TLR | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 3 (0.6) | 0 (0) | 1 (1.4) | 3 (0.7) | 1 (0.4) |
| TVR | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 8 (1.5) | 2 (1.6) | 2 (2.8) | 7 (1.6) | 2 (0.7) |
| CD-TLR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.6) | 2 (1.6) | 0 (0) | 2 (0.5) | 0 (0) |
| Non-CD-TLR | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 5 (1.0) | 0 (0) | 2 (2.8) | 5 (1.2) | 2 (0.7) |
| Non-target vessel revascularisation | 3 (0.6) | 1 (0.8) | 1 (1.4) | 2 (0.5) | 1 (0.4) | 10 (1.9) | 2 (1.6) | 2 (2.8) | 9 (2.1) | 6 (2.2) |
| Post-procedure occlusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bleeding or vascular event | 3 (0.6) | 1 (0.8) | 0 (0) | 2 (0.5) | 1 (0.4) | 5 (1.0) | 2 (1.6) | 0 (0) | 3 (0.7) | 1 (0.4) |
| Any stent thrombosis | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Definite stent thrombosis | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Probable stent thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Data are n (%). CD-TLR: clinically driven target lesion revascularisation; CD-TVR: clinically driven target vessel revascularisation; MI: myocardial infarction; TLF: target lesion failure; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction; TVR: target vessel revascularisation | ||||||||||
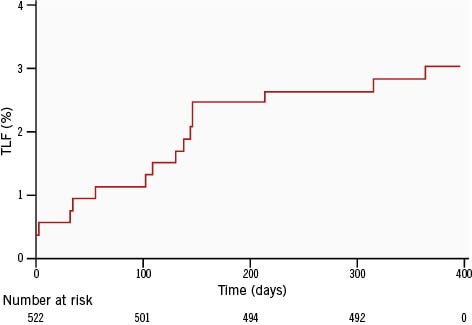
Figure 1. Kaplan-Meier curve showing target lesion failure up to 12-month follow-up in the overall population. TLF: target lesion failure
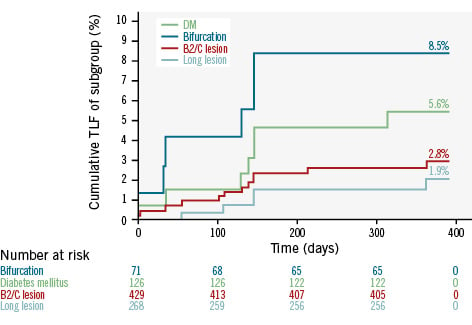
Figure 2. Kaplan-Meier curves for target lesion failure up to 12-month follow-up in the subgroups with diabetes mellitus, bifurcation lesions, type B2/C lesions, and long lesions (>20 mm). DM: diabetes mellitus; TLF: target lesion failure
Discussion
The S-FLEX Russia registry is a report on the first Russian experience of the Supraflex Cruz SES in a real-world, all-comers population who underwent PCI. The major findings of the present registry can be summarised as follows: (i) at 12 months, the primary endpoint of TLF was 3.1%; (ii) overall definite/probable stent thrombosis was 0.2%, reported within 30 days of the procedure; (iii) in high-risk subgroups, including patients with diabetes, bifurcation lesions, and long lesions (>20 mm), no incidence of stent thrombosis was reported at 12-month follow-up.
Technical iterations of the latest-generation DES have improved the arterial healing pattern1011. The unique LDZ link design in the Supraflex Cruz SES, along with long connectors and thinner struts, offers exceptional trackability, aids flexibility, and provides good structural support with better push force through complex lesions and tortuous coronary arteries512. Consequently, these design characteristics collectively enhance stent integrity and radial strength, and resist longitudinal stent compression and foreshortening13. In addition, thinner struts with a biodegradable-polymer coating potentially prevent the risk of acute/chronic inflammation, reduce thrombogenicity and vessel wall injury, and accelerate re-endothelialisation and strut coverage, which ultimately decreases the need of revascularisation41415. At 12-month follow-up, the TLF rate in the present registry was 3.1%, including 1.5% cardiac death, 0.4% CD-TLR, and 1.1% TVMI. In line with the findings of the present registry, the S-FLEX UK-II registry reported a TLF rate of 2.3% with the Supraflex Cruz SES at 12-month follow-up among its UK population16. Similarly, the T-FLEX registry reported a TLF rate of 3.8% with the Supra family of SES in an Indian population at 12-month follow-up5. On the other hand, the results from the BIOFLOW-III Canada registry (2.8%), Thailand Orsiro registry (5.3%), BIOFLOW-IV Japanese registry (4.2%) and BIOFLOW-III Italian Satellite registry (4.6%) reported numerically higher rates of TLF with Orsiro SES (Biotronik) in all-comers patients with CAD, at 12-month follow-up231718.
Some of the factors associated with the incidence of stent thrombosis include an underexpanded stent, endothelial disruption or an injury caused by edge dissection, or delayed healing with DES and hypercoagulability19. The rate of definite stent thrombosis in this S-FLEX Russia registry was just 0.2% (one case), and it was reported within 30 days of the procedure. Notably, between 30 days and 12 months no incidence of stent thrombosis was reported in the overall population. The low incidence of stent thrombosis in our study might be attributed to early arterial healing and almost complete strut coverage, as reported in the SiBi optical coherence tomography (OCT) study (91.26% strut coverage at 35.3±5 days of OCT follow-up) and the TAXCO study (97.6% strut coverage at 6-month OCT follow-up) on the Supra family of SES1011. This finding of the present Russia registry is consistent with the S-FLEX UK-II registry, which reported 0.3% of overall stent thrombosis with the Supraflex Cruz SES among the UK population16. In contrast, the BIOFLOW-III Canada registry (0.4%), Thailand Orsiro registry (1.3%), BIOFLOW-IV Japanese registry (0.8%) and BIOFLOW-III Italian Satellite registry (0.5%) reported numerically higher rates of stent thrombosis at 12-month follow-up231718.
In the S-FLEX Russia registry, at 12-month follow-up, the TLF rates with the Supraflex Cruz SES were favourable among patients in high-risk subgroups, including diabetes (5.6%), bifurcation lesions (8.5%), type B2/C lesions (2.8%), and long lesions of >20 mm (1.9%), which were comparable with the S-FLEX UK-II registry that reported TLF rates of 6.2% in patients with diabetes, 1.8% in bifurcation lesions, 2.5% in type B2/C lesions and 2.7% in long lesions (>20 mm) with the Supraflex Cruz SES in a UK population16. Likewise, a pooled analysis from two Indian registries reported similar rates of TLF with the Supra family of SES in patients with diabetes mellitus (6.9%), multivessel disease (6.4%), total occlusion (5.2%), long lesions (6.6%) and small vessels (6.1%)20. These favourable results with the Supraflex Cruz SES have demonstrated effectiveness, even in patients in high-risk subgroups.
Variability in event rates has been noted across various registries encompassing real-world, all-comers patients from diverse geographical regions around the globe. In addition to device characteristics, several other factors − such as patient and lesion complexity, endpoint definitions, functional assessment for myocardial ischaemia, event adjudication, event reporting, data monitoring, and medical therapy − may also influence the reported event rates in patients with CAD across various registries. Therefore, it is imperative to interpret the discrepancies in TLF rates while considering all these factors and their potential influence on the reported outcomes.
Limitations
There are certain limitations of this study. First, this was a non-randomised study and lacked a direct comparison to other contemporary DES, which could have provided better insights into the outcomes. Second, the coronary lesions were assessed through visual estimation, and no intracoronary imaging modalities were used, which could otherwise have provided details on strut coverage and apposition of the DES. Third, follow-up was only conducted up to 12 months; longer-term follow-up is necessary to evaluate the safety and efficacy of the DES.
Conclusions
The S-FLEX Russia registry demonstrates the excellent safety and efficacy of the biodegradable polymer-coated Supraflex Cruz SES in real-world, all-comers Russian patients with CAD. The Supraflex Cruz SES showed excellent device and procedural success with low rates of TLF and stent thrombosis at 12-month follow-up, including in prespecified high-risk subgroups.
Conflict of interest statement
The authors have no conflicts of interest to declare.Impact on daily practicePercutaneous coronary intervention with a drug-eluting stent is the most prevalent revascularisation treatment approach for coronary artery disease (CAD). The biodegradable polymer-coated Supraflex Cruz sirolimus-eluting stent (SES) demonstrated excellent clinical performance in an all-comers population with CAD in Russia. Even in high-risk subgroups, including those with diabetes, bifurcation lesions, B2/C lesions and long lesions (>20 mm), the Supraflex Cruz SES showed favourable outcomes. The Supraflex Cruz SES is thus safe and effective in patients with CAD in Russia.

