The growing prevalence of diabetes poses a significant global health challenge. In 2021, it affected approximately 537 million individuals and resulted in over 6.7 million deaths1. In Bangladesh, a 2018 study found that 13.75% of adults over 35 years have diabetes mellitus (DM), with prevalence rates increasing to 38% in those aged 65-74 years2. DM substantially raises the risk of cardiovascular events, particularly through coronary artery calcification and endothelial dysfunction, making cardiovascular disease the leading cause of mortality in this population3.
The management of left main coronary artery (LMCA) disease in diabetic patients has unique challenges. Revascularisation, guided by the SYNTAX score and patient-specific factors, is critical4. Both percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are recommended for patients with a low SYNTAX score (≤22), while CABG is preferred for intermediate (23-32) and complex cases (≥33)5. Advances in stent technology and pharmacotherapy have improved PCI outcomes, highlighting the need for personalised treatment strategies6. Approximately 25% of patients undergoing PCI have pre-existing DM, which correlates with a higher frequency of repeat revascularisation and lower long-term survival rates compared to non-diabetic patients78. However, second-generation drug-eluting stents (DES), like the Resolute zotarolimus-eluting stent (ZES; Medtronic), have significantly improved outcomes by reducing thrombosis, restenosis, and the need for revascularisation9. The SYNTAX II trial further demonstrated the efficacy of thin-strut bioresorbable-polymer DES in lowering major adverse cardiovascular events (MACE) compared to CABG in the SYNTAX I trial9.
This study aimed to evaluate all-cause mortality, cardiac-related deaths, and major predictors of cardiac death among patients undergoing LMCA revascularisation with the Resolute ZES, with a focus on the differences between diabetic and non-diabetic populations.
Methods
Study design and population
This retrospective study was conducted at a tertiary care cardiac hospital in Bangladesh between 2010 and 2019. During this period, a total of 1,940 left main (LM) PCIs were carried out. Of these, 51.5% (999) involved the use of ZES, 33.9% (658) everolimus-eluting stents, 7.5% (145) sirolimus-eluting stents, 4.0% (77) biolimus-eluting stents, 2.0% (38) paclitaxel-eluting stents, and 1.2% (23) bare metal stents. This was a single-centre, multioperator study, and the choice of the stent was at the discretion of the operators. Out of 1,940 LM PCI patients, 55.8% (1,082) patients were diabetic.
A cohort of 999 participants who had undergone LM PCI with the deployment of the Resolute ZES was initially reviewed. However, only 884 patients with complete follow-up data were included (Figure 1). The primary reason for excluding the remaining 115 patients was the unavailability of follow-up information.
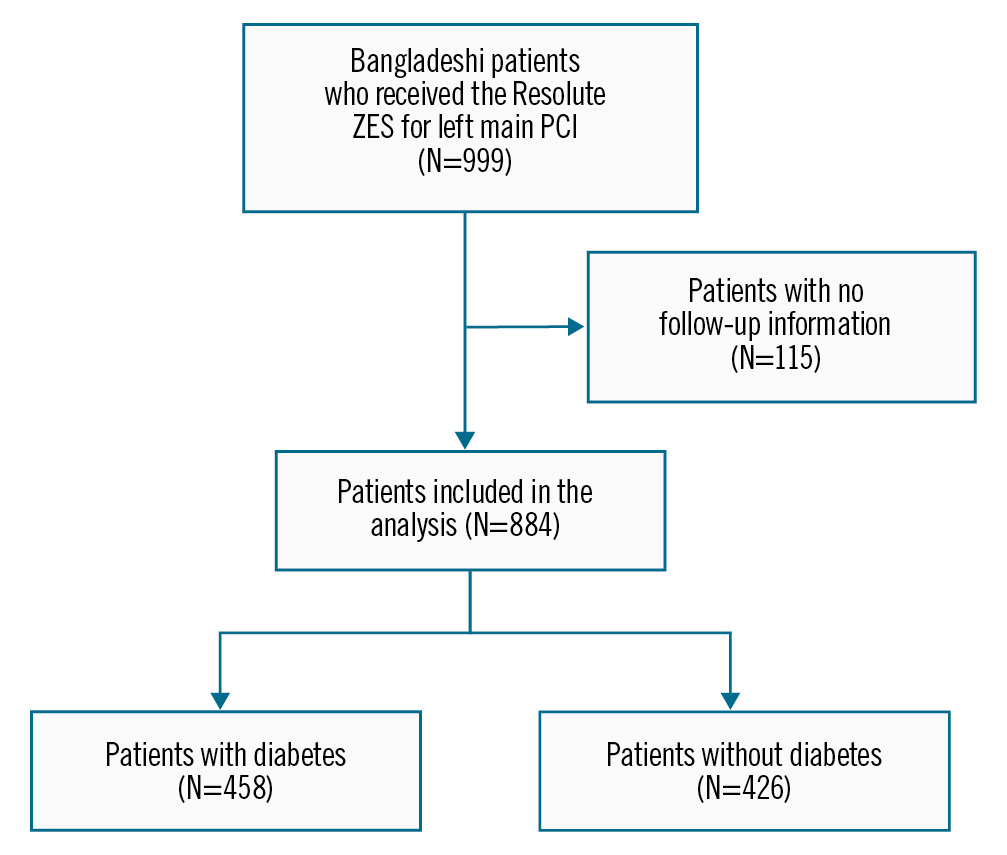
Figure 1. Patient selection criteria. PCI: percutaneous coronary intervention; ZES: zotarolimus-eluting stent
Objectives
The primary objective was to assess all-cause mortality and cardiac death rates in subjects with and without diabetes who underwent LM PCI using the Resolute ZES. The primary endpoint of this study was the difference in mortality between the two groups (diabetes vs non-diabetes). The secondary objective was to identify major predictors of cardiac death among baseline characteristics, lesion features, and procedural details.
Data collection and descriptive analysis
Data on patient demographics, risk factors (e.g., family history, hypertension, smoking, and dyslipidaemia), and lesion/procedure characteristics were collected. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m². New diabetes diagnoses were based on plasma glucose criteria, either the fasting plasma glucose (FPG) value or the 2-hour plasma glucose (PG) value during a 75 g oral glucose tolerance test (OGTT), or A1C criteria in an individual not known to be diabetic. Diabetes was confirmed if one criterion was met: FPG ≥126 mg/dL (7.0 mmol/L), 2-hour PG ≥200 mg/dL (11.1 mmol/L) during OGTT, A1C ≥6.5%, or a patient with classic symptoms of hyperglycaemia or hyperglycaemic crisis with a random PG ≥200 mg/dL (11.1 mmol/L). Diabetes was also confirmed if a patient was on antidiabetic medication or diet therapy alone.
Comprehensive data collection facilitated a descriptive analysis of subject demographics and clinical characteristics. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges depending on the normality of the distribution. Categorical variables are summarised as counts and percentages.
Statistical analysis
For continuous variables, group comparisons were made using the Student’s t-test or Wilcoxon’s rank-sum test, based on data distribution. Categorical data were analysed using the chi-squared test or Fisher’s exact test, as appropriate. Kaplan-Meier curves were plotted to illustrate time-to-death analyses, with the log-rank test employed to discern statistically significant differences between patient cohorts. Cox regression models were utilised for both univariate and multivariate analyses to examine potential predictors of mortality, generating hazard ratios (HRs) with 95% confidence intervals (CIs). The proportionality of hazards was verified using Schoenfeld residuals. Variables with p-values<0.10 in the univariate analyses were subsequently incorporated into the multivariate analysis to assess predictors of cardiac death. A stepwise selection process was adopted, with entry criteria set at a p-value<0.30 and stay criteria at a p-value<0.05, to determine the most significant predictors among baseline, lesion, and procedural characteristics. The primary endpoint, the mortality difference between groups, was assessed using the log-rank test and Cox proportional hazards models.
No missing data imputation was performed. The Statistical Analysis System software (SAS; version 9.4 [SAS Institute]) was used to carry out two-tailed tests; results were considered statistically significant at p<0.05.
Results
A total of 884 patients with complete follow-up information were included in the descriptive and primary analyses, according to patient disposition. A total of 115 patients had no/incomplete follow-up information; of these, 58.26% (67 patients) were diabetic.
Demographic and baseline characteristics
The study cohort comprised 884 subjects who underwent PCI with the Resolute ZES, including 458 patients (51.8%) with diabetes and 426 patients (48.2%) without diabetes. The average age was slightly higher in the diabetes group than in the non-diabetes group (57±10 vs 56±12 years; p=0.031). A smaller proportion of subjects with diabetes were ≤50 years old compared to their non-diabetes counterparts (24.9% vs 36.2%; p=0.003). Sex distribution was similar across groups, with 81.1% of males overall. The mean body mass index (BMI) was comparable between groups (25.2±3.6 kg/m2 for the total cohort; p=0.394). CKD was present in 43.8% of the total cohort and in similar proportions in both groups (p=0.839).
There were significantly more patients with hypertension in the diabetes group than in the non-diabetes group (80.1% vs 73.2%; p=0.015). Smoking rates were similar across both groups (51.0%), as was the prevalence of dyslipidaemia (68.6% in the total cohort; p=0.146) and family history of ischaemic heart disease (IHD; 21.0%; p=0.664). The distribution of other diagnoses did not significantly differ between the groups (Table 1).
Table 1. Demographic, baseline, and procedural characteristics for groups of patients with and without diabetes.
| Parameter | Total(N=884) | Diabetes(N=458) | No diabetes(N=426) | p-value |
|---|---|---|---|---|
| Age, years | 57±11 | 57±10 | 56±12 | 0.031 |
| ≤50 | 268 (30.3) | 114 (24.9) | 154 (36.2) | 0.003 |
| 51-58 | 210 (23.8) | 121 (26.4) | 89 (20.9) | |
| 59-64 | 182 (20.6) | 99 (21.6) | 83 (19.5) | |
| ≥65 | 224 (25.3) | 124 (27.1) | 100 (23.5) | |
| Sex | 0.670 | |||
| Male | 717 (81.1) | 369 (80.6) | 348 (81.7) | |
| Female | 167 (18.9) | 89 (19.4) | 78 (18.3) | |
| BMI, kg/m2 | 25.2±3.6 | 25.3±3.5 | 25.0±3.7 | 0.394 |
| CKD | 387 (43.8) | 202 (44.1) | 185 (43.4) | 0.839 |
| Risk factors | ||||
| Hypertension | 679 (76.8) | 367 (80.1) | 312 (73.2) | 0.015 |
| Smoking | 451 (51.0) | 234 (51.1) | 217 (50.9) | 0.964 |
| Dyslipidaemia | 606 (68.6) | 324 (70.7) | 282 (66.2) | 0.146 |
| Family history of IHD | 186 (21.0) | 99 (21.6) | 87 (20.4) | 0.664 |
| Diagnosis | 0.117 | |||
| ACS | 313 (35.4) | 153 (33.4) | 160 (37.6) | |
| CCS | 571 (64.6) | 305 (66.6) | 266 (62.4) | |
| LVEF, % | 51.7±9.7 | 51.4±10.1 | 52.0±9.3 | 0.494 |
| Laboratory value of creatinine, mg/dL | 1.3±0.7 | 1.3±0.8 | 1.2±0.6 | 0.422 |
| CAG findings | 0.621 | |||
| Isolated LMCA disease | 110 (12.4) | 56 (12.2) | 54 (12.7) | |
| LMCA disease with SVD | 258 (29.2) | 126 (27.5) | 1,332 (31.0) | |
| LMCA disease with DVD | 321 (36.3) | 169 (36.9) | 152 (35.7) | |
| LMCA disease with TVD | 195 (22.1) | 107 (23.4) | 88 (20.7) | |
| Involved location | 0.816 | |||
| Distal | 774 (87.6) | 402 (87.8) | 372 (87.3) | |
| Ostial | 87 (9.8) | 43 (9.4) | 44 (10.3) | |
| Shaft | 23 (2.6) | 13 (2.8) | 10 (2.3) | |
| Stenting strategy | 0.287 | |||
| Double | 200 (22.6) | 97 (21.2) | 103 (24.2) | |
| Single | 684 (77.4) | 361 (78.8) | 323 (75.8) | |
| Technique | 0.853 | |||
| Culotte | 66/200 (33.0) | 31/97 (32.0) | 35/103 (34.0) | |
| DK crush | 8/200 (4.0) | 5/97 (5.2) | 3/103 (2.9) | |
| M crush | 22/200 (11.0) | 10/97 (10.3) | 12/103 (11.7) | |
| TAP | 104/200 (52.0) | 51/97 (52.6) | 53/103 (51.5) | |
| Lesion type | 0.525 | |||
| Protected | 61 (6.9) | 34 (7.4) | 27 (6.3) | |
| Unprotected | 823 (93.1) | 424 (92.6) | 399 (93.7) | |
| Bifurcation | 774 (87.6) | 402 (87.8) | 372 (87.3) | |
| Access route | 0.736 | |||
| Femoral | 872 (98.6) | 453 (98.9) | 419 (98.4) | |
| Radial | 9 (1.0) | 4 (0.9) | 5 (1.2) | |
| Snuffbox | 3 (0.3) | 1 (0.2) | 2 (0.5) | |
| Predilatation | 824 (93.2) | 431 (94.1) | 393 (92.3) | 0.274 |
| Post-dilatation | 862 (97.5) | 446 (97.4) | 416 (97.7) | 0.795 |
| Final kissing | 356 (40.3) | 192 (41.9) | 164 (38.5) | 0.300 |
| FFR | 7 (0.8) | 4 (0.9) | 3 (0.7) | 0.777 |
| Data are n (%), n/N (%), or mean±SD. ACS: acute coronary syndrome; BMI: body mass index; CAG: coronary angiography; CCS: chronic coronary syndrome; CKD: chronic kidney disease; DK: double kissing; DVD: double-vessel disease; FFR: fractional flow reserve; IHD: ischaemic heart disease; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; SD: standard deviation; SVD: single-vessel disease; TAP: T and small protrusion; TVD: triple-vessel disease | ||||
Procedural characteristics
In the study, a total of 230 procedures were guided by intravascular ultrasound (IVUS), with all patients undergoing IVUS as part of the intervention. Optical coherence tomography was not used. On average, the left ventricular ejection fraction was 51.7±9.7% across the cohort. Isolated LMCA disease was present in 12.4% of the patients, with similar numbers in both groups (p=0.621). LMCA disease in conjunction with single-vessel disease, double-vessel disease, and triple-vessel disease was found in 29.2%, 36.3%, and 22.1% of the cohort, respectively. The majority of lesions were located distally (87.6%), with similar distributions in both the diabetes and non-diabetes patient groups (p=0.816). Regarding the revascularisation strategy, 22.6% of interventions used a double-stenting approach, while the majority (77.4%) were single-stent procedures, with no significant difference between the groups (p=0.287). The culotte technique was used in 33.0% of patients (66/200), the double-kissing (DK) crush technique in 4.0% (8/200), the M crush technique in 11.0% (22/200), and the T and small protrusion (TAP) technique in 52.0% of patients (104/200). Protected lesions were noted in 6.9% of the cohort, and unprotected lesions were more common, representing 93.1% of cases. The prevalence of bifurcation lesions was high (87.6%), emphasising the complexity of the interventions. The femoral route was the predominant access site used (98.6%), followed by the radial (1.0%) and snuffbox (0.3%) approaches. Predilatation was carried out in 93.2% of cases, and post-dilatation was employed in 97.5%, indicating a high rate of complete lesion preparation and stent optimisation. All patients received the Resolute ZES (Table 1).
In our cohort of 884 patients, 77.4% (684/884) underwent a single-stent strategy during left main PCI, while 22.6% (200/884) were treated with a two-stent approach. Among the diabetic patients, 78.8% (361/458) received single-stent treatment, compared to 75.8% (323/426) in the non-diabetes group. For the two-stent approach, the proportions were 21.2% (97/458) in the diabetic population and 24.2% (103/426) in the non-diabetic group. Statistical analysis showed no significant difference between the groups in terms of the choice of stenting strategy (p=0.287). These results suggest that both the diabetic and non-diabetic populations were similarly treated in terms of single- versus two-stent strategies.
In-hospital endpoints
No differences between the two groups were apparent with respect to the incidences of in-hospital events such as haematoma, repeat revascularisation, arrhythmia, heart failure, stroke, and death. Procedural success was also similar between the groups (Table 2).
Table 2. In-hospital endpoints for groups of patients with and without diabetes.
| Endpoint | All (n=884) | Diabetes (n=458) | No diabetes (n=426) |
|---|---|---|---|
| Haematoma | 21 (2.4) | 8 (1.8) | 13 (3.1) |
| Repeat revascularisation | 8 (0.9) | 4 (0.9) | 4(0.9) |
| CABG | 3 (0.3) | 1 (0.2) | 2 (0.5) |
| PCI | 5 (0.6) | 3 (0.7) | 2 (0.5) |
| Arrhythmia | 13 (1.5) | 5 (1.1) | 8 (1.9) |
| Heart failure | 2 (0.2) | 0 | 2 (0.5) |
| Stroke | 3 (0.3) | 1 (0.2) | 2 (0.5) |
| Death | 5 (0.6) | 3 (0.7) | 2 (0.5) |
| Procedural success | 838 (94.8) | 439 (95.9) | 399 (93.7) |
| Data are n (%). CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention | |||
All-cause mortality and cardiac death
The cumulative incidences of all-cause mortality at 1 and 6 years were 2.6% (95% CI: 1.7-4.0%) and 8.5% (95% CI: 6.0-11.9%), respectively. The incidence rates of cardiac death at 1 and 6 years were 2.2% (95% CI: 1.3-3.5%) and 6.9% (95% CI: 4.6-10.2%), respectively. No model was built for all-cause death due to the relatively low frequency of events, which made it statistically impractical to create a robust predictive model for this outcome.
Upon comparing the incidence curves over a 6-year period, no statistically significant difference in all-cause mortality between the diabetes and non-diabetes groups was observed (p=0.67) (Figure 2A). Similarly, cardiac death incidences were also similar (p=0.61) between the groups (Figure 2B).
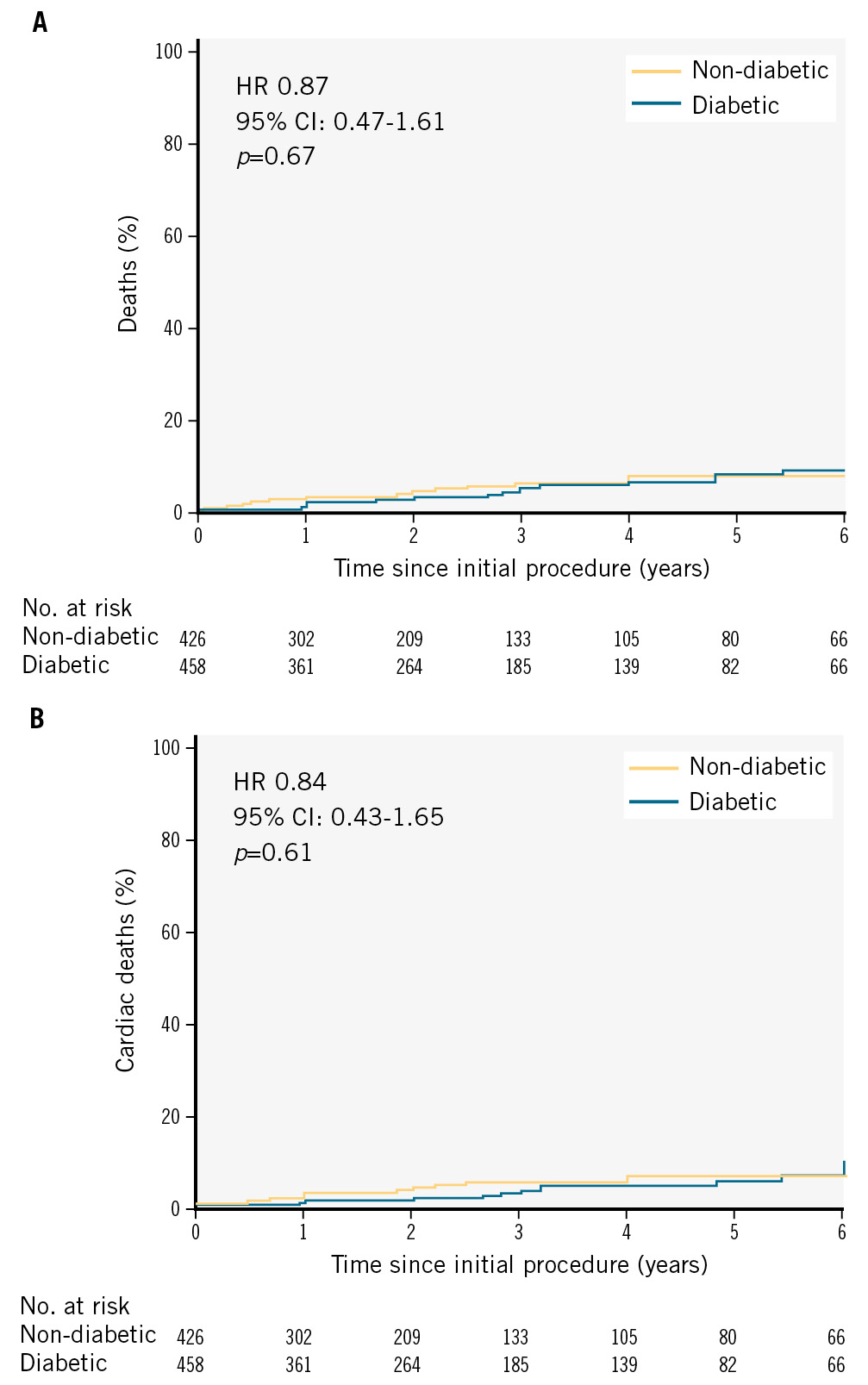
Figure 2. Kaplan-Meier survival curves for all-cause mortality and cardiac death during follow-up. A) All-cause mortality during follow-up. B) Cardiac deaths during follow-up. CI: confidence interval; HR: hazard ratio
Mortality outcomes
The cumulative all-cause mortality rates over the 2-year and 3-year follow-up periods were analysed using Kaplan-Meier survival curves. Figure 3A shows the cumulative mortality over the first 2 years following the initial procedure, revealing a low mortality rate throughout the period. A similar trend was observed over 3 years, as displayed in Figure 3B, where the mortality rate remained consistently low.
When comparing mortality outcomes between diabetic and non-diabetic patients, the 2-year Kaplan-Meier curves (Figure 4A) showed no significant difference in survival rates (p=0.46). Likewise, the 3-year survival rates between the two groups, as illustrated in Figure 4B, demonstrated no statistically significant difference (p=0.39).
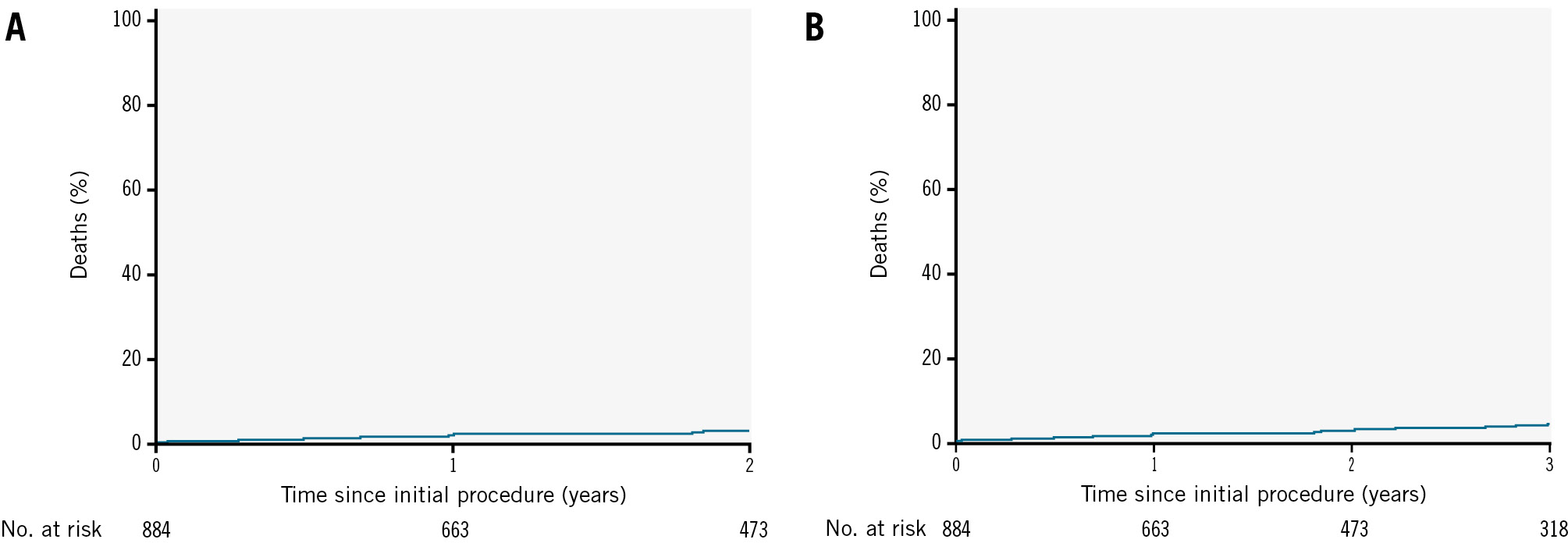
Figure 3. Kaplan-Meier survival curves for mortality. A) Cumulative mortality over 2 years. B) Cumulative mortality over 3 years.
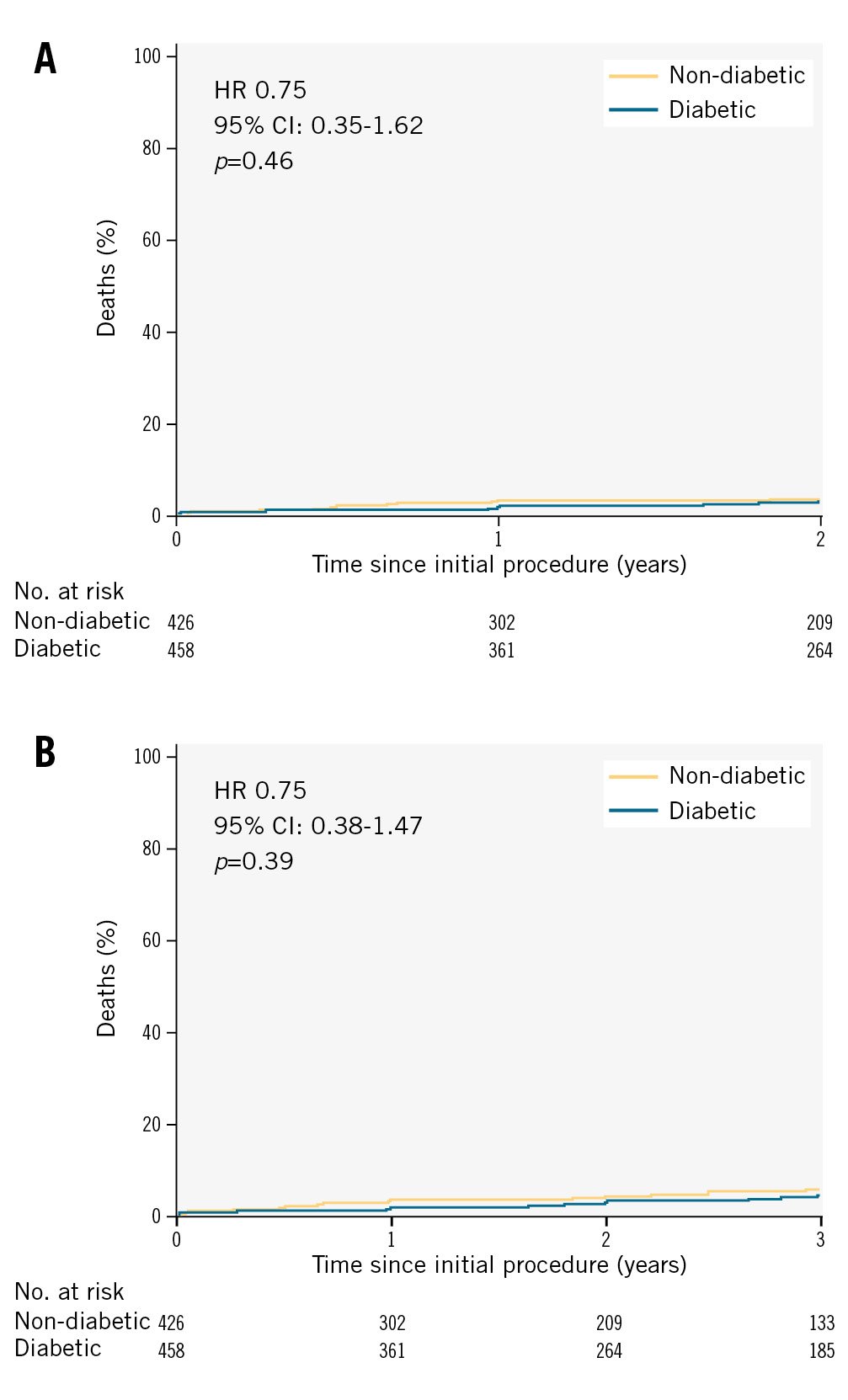
Figure 4. Kaplan-Meier survival curves: comparison between diabetic and non-diabetic patients. A) 2-year mortality. B) 3-year mortality. CI: confidence interval; HR: hazard ratio
Cardiac death
The analysis of cardiac death over the 2-year period, presented in Figure 5A, indicates a stable and low cumulative incidence. Similarly, the cumulative incidence of cardiac death over 3 years (Figure 5B) remained low and without a notable increase.
When stratified by diabetic status, the Kaplan-Meier curves for cardiac death over 2 years (Figure 6A) and 3 years (Figure 6B) revealed no significant differences between the diabetes and non-diabetes groups (p=0.15 and p=0.22, respectively), further supporting that diabetic status was not associated with an increased risk of cardiac death during the follow-up periods.
In our cohort, both protected and unprotected LM disease cases were included. The protected group consisted of 61 patients, with a mortality rate of 1.6% (1/61). In contrast, the unprotected group comprised 823 patients, with a higher mortality rate of 4.7% (39/823). These findings suggest that patients with unprotected LM disease have a higher mortality risk compared to those with protected LM disease.
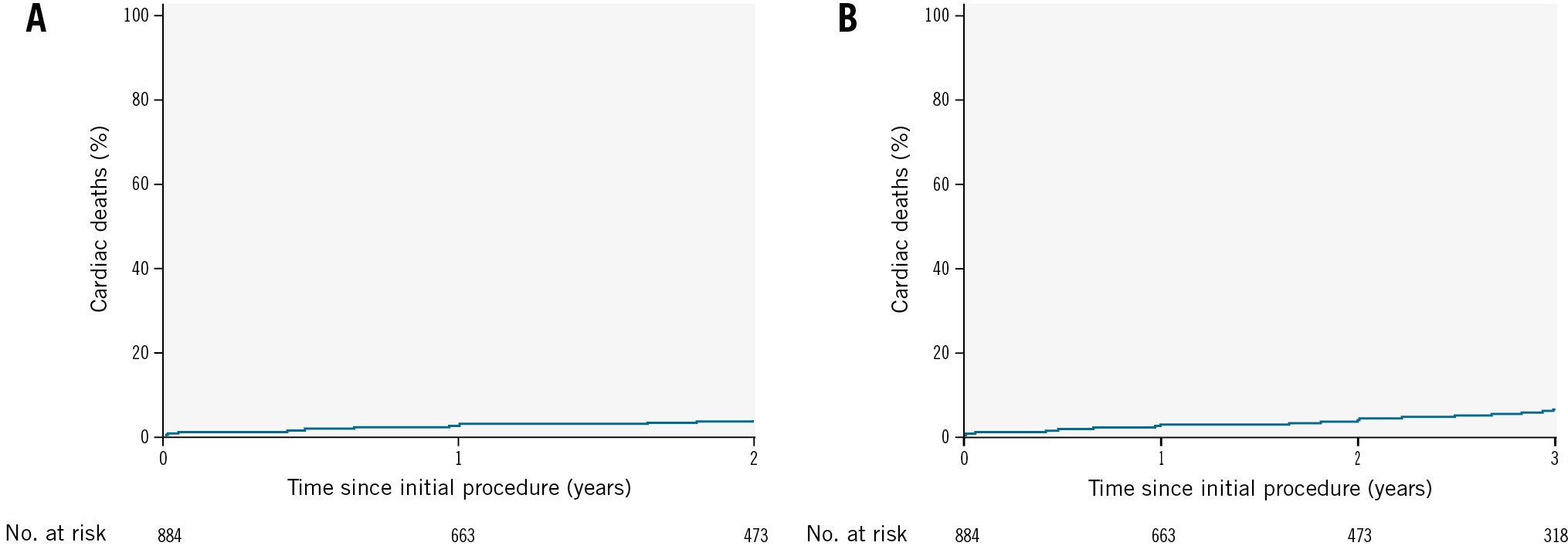
Figure 5. Kaplan-Meier survival curves for cardiac death. A) Cardiac deaths over a 2-year period after the index procedure. B) Cardiac deaths over a 3-year period after the index procedure.
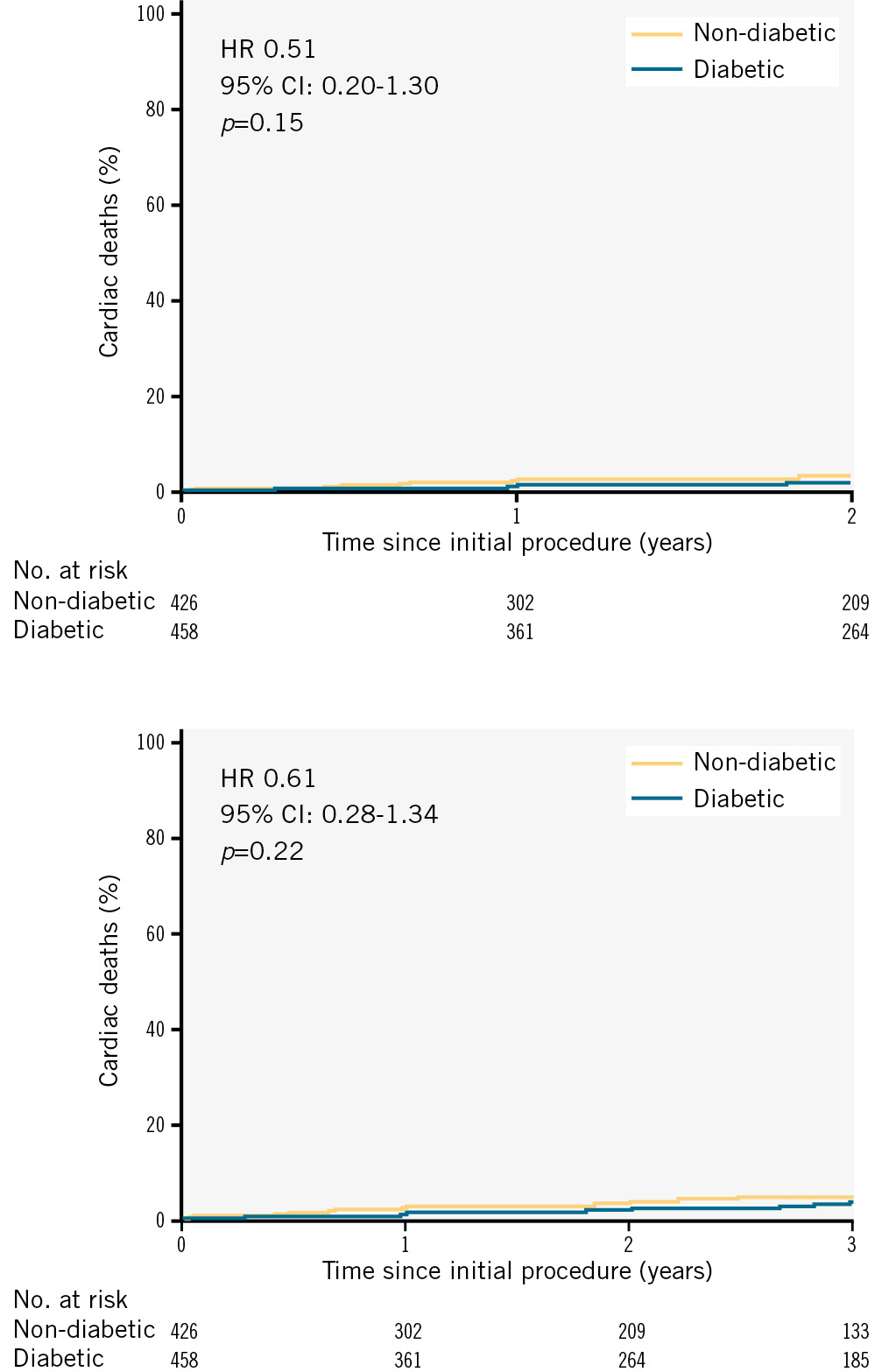
Figure 6. Kaplan-Meier survival curves for cardiac death stratified by diabetic status. A) Survival over 2 years. B) Survival over 3 years. CI: confidence interval; HR: hazard ratio
Predictors of cardiac death
Table 3 presents the analysis of predictors for cardiac death in patients undergoing left main PCI. Age was found to be a significant predictor of mortality, with the risk of cardiac death increasing by approximately 8-9% for each additional year of age (HR 1.09; p<0.001). Elevated creatinine levels (≥1.5837 mg/dL) were also strongly associated with a higher mortality risk, with hazard ratios ranging from 3.67 to 4.45 across models (p=0.001-0.002), indicating that impaired kidney function plays a critical role in patient outcomes.
Post-dilatation of the stent showed a protective effect, reducing the risk of cardiac death by approximately 90% in the multivariate model (HR 0.10-0.12; p=0.001), highlighting the importance of stent optimisation during the procedure.
The Medina classification, particularly the 1,0,0 configuration, significantly increased the risk of cardiac death, with a hazard ratio of 18.56 in the multivariate model (p=0.005). Other Medina classifications, such as 1,0,1; 1,1,0; and 1,1,1, did not show statistically significant associations with mortality.
Interestingly, other factors such as sex, presence of diabetes, BMI, hypertension, smoking, and dyslipidaemia were not found to be significant predictors of cardiac death, with p-values exceeding 0.05. This suggests that these common clinical characteristics may not independently contribute to mortality in this patient cohort.
In summary, key predictors of cardiac death included age, creatinine levels, and post-dilatation, with the Medina class 1,0,0 showing a particularly high risk. These findings underscore the need for careful patient and procedural management to optimise outcomes in high-risk populations undergoing left main PCI.
Table 3. Univariate and multivariate analysis of predictors of cardiac death, including Medina classification.
| Predictor | Univariate HR (95% CI) | Univariate p-value | Multivariate HR (95% CI) | Multivariate p-value | Multivariate HR (95% CI) (without Medina classification) | Multivariate p-value(without Medina classification) |
|---|---|---|---|---|---|---|
| Age | 1.08 (1.04-1.11) | <0.001 | 1.09 (1.06-1.13) | <0.001 | 1.09 (1.05-1.12) | <0.001 |
| Male sex | 0.57 (0.27-1.23) | 0.154 | ||||
| Diabetes | 0.85 (0.43-1.66) | 0.630 | ||||
| BMI ≥30 kg/m2 | 0.36 (0.05-2.60) | 0.309 | ||||
| CKD | 3.00 (1.46-6.15) | 0.003 | ||||
| CCS diagnosis | 1.09 (0.56-2.15) | 0.794 | ||||
| Hypertension | 0.77 (0.38-1.57) | 0.470 | ||||
| Smoking | 0.78 (0.40-1.53) | 0.475 | ||||
| Dyslipidaemia | 1.07 (0.43-2.65) | 0.879 | ||||
| Family history of IHD | 0.36 (0.09-1.54) | 0.169 | ||||
| LVEF | 0.99 (0.95-1.02) | 0.382 | ||||
| LVEF ≤35% | 1.34 (0.32-5.61) | 0.685 | ||||
| Hb | 0.83 (0.64-1.07) | 0.142 | ||||
| Creatinine ≥1.5837 mg/dL | 3.67 (1.63-8.26) | 0.002 | 4.45 (1.80-11.02) | 0.001 | 4.33 (1.76-10.64) | 0.001 |
| Involved vessel: LAD | 1.47 (0.68-3.20) | 0.330 | ||||
| Lesion percentage LAD | 1.00 (0.95-1.06) | 0.931 | ||||
| Involved vessel: diagonal | 1.87 (0.25-13.75) | 0.540 | ||||
| LCx vessel | 1.53 (0.71-3.30) | 0.276 | ||||
| Involved vessel: OM | 2.17 (0.29-15.99) | 0.448 | ||||
| RCA vessel | 1.75 (0.78-3.94) | 0.176 | ||||
| Double-stenting strategy | 1.55 (0.72-3.33) | 0.259 | ||||
| Bifurcation lesion | 0.83 (0.34-2.01) | 0.683 | ||||
| Predilatation | 0.79 (0.28-2.27) | 0.665 | ||||
| Post-dilatation | 0.28 (0.09-0.92) | 0.035 | 0.10 (0.03-0.39) | 0.001 | 0.12 (0.03-0.42) | 0.001 |
| Final kissing | 1.12 (0.57-2.20) | 0.752 | ||||
| LM length ≥26 mm | 1.77 (0.88-3.54) | 0.109 | ||||
| LM diameter | 0.41 (0.16-1.08) | 0.070 | ||||
| Check CAG | 0.52 (0.12-2.18) | 0.372 | ||||
| LM with DVD vs isolated LMCA | 0.36 (0.11-1.19) | 0.094 | ||||
| LM with SVD vs isolated LMCA | 0.90 (0.33-2.43) | 0.831 | ||||
| LM with TVD vs isolated LMCA | 1.58 (0.59-4.23) | 0.362 | ||||
| Medina class 1,0,0 | 7.41 (1.00-54.68) | 0.049 | 18.56 (2.40-143.26) | 0.005 | ||
| Medina class 1,0,1 | 1.76 (0.24-12.99) | 0.577 | ||||
| Medina class 1,1,0 | 0.68 (0.32-1.46) | 0.322 | ||||
| Medina class 1,1,1 | 1.21 (0.58-2.55) | 0.611 | ||||
| Ostial vs distal | 1.07 (0.37-3.04) | 0.906 | ||||
| Shaft vs distal | 1.62 (0.39-6.83) | 0.510 | ||||
| BMI: body mass index; CAG: coronary angiography; CCS: chronic coronary syndrome; CI: confidence interval; DVD: double-vessel disease; Hb: haemoglobin; HR: hazard ratio; IHD: ischaemic heart disease; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; OM: obtuse marginal; RCA: right coronary artery; SVD: single-vessel disease; TVD: triple-vessel disease | ||||||
Discussion
The management of LMCA disease in diabetic patients remains a complex clinical challenge due to the elevated atherosclerotic burden and increased numbers of lipid-rich plaques typically observed in this population10. Recent advancements, such as intracoronary physiology assessment, intravascular imaging guidance, less invasive revascularisation, and thin-strut DES, have firmly established PCI as a viable alternative to CABG, with outcomes comparable to CABG1112.
This study compared the effectiveness of PCI with the Resolute ZES in treating LMCA disease in patients with and without diabetes. The baseline characteristics reveal that patients in the non-diabetes group were marginally younger and had a lower prevalence of hypertension than those in the diabetes group. Several other studies report similar trends, where patients with diabetes have a higher prevalence of comorbidities than those without diabetes813. However, other demographic factors, BMI, and clinical diagnoses did not differ significantly between the groups. A considerable portion of patients exhibited multivessel disease, which possibly had implications for clinical decision-making and procedural approaches. The procedural strategies, including stenting techniques, were diverse, with the application of double-stenting strategies, such as culotte, DK crush, M crush, and TAP, reflecting the complexity of interventions required for bifurcation lesions. Interestingly, the choice of stenting technique did not significantly differ between the diabetes and non-diabetes groups. The decision-making process for selecting the appropriate stenting technique needs further exploration to understand the effect of a lesion’s anatomical characteristics, including its location within the LMCA.
However, despite the overall success, the occurrence of in-hospital endpoints such as haematoma, arrhythmia, and repeat revascularisation, although low, emphasises the continual need for optimisation of both patient selection and procedural protocols to minimise such events.
No significant differences were noted in all-cause or cardiac death rates between diabetic and non-diabetic patients treated with the Resolute ZES. This is consistent with current literature that suggests comparable outcomes between PCI and CABG in specific patient subsets. Similarly, subgroup analyses in the EXCEL trial indicated that PCI outcomes are similar to CABG outcomes in patients with diabetes and LMCA disease14. The 10-year report from the MAIN-COMPARE registry revealed comparable long-term risks of mortality and serious outcomes between PCI and CABG in patients with diabetes15. However, some studies have reported higher in-hospital deaths, occurrences of non-fatal myocardial infarctions (MIs), and revascularisation rates among individuals with diabetes undergoing PCI8.
This study identified age, CKD, elevated creatinine levels, and post-dilatation as independent predictors of cardiac death in patients with LMCA disease. Baseline kidney function and low postprocedural flow have been reported as factors contributing to cardiac death16. The association between creatinine levels and cardiac death emphasises the critical need for kidney function monitoring. Post-dilatation is an integral part of stent deployment as it ensures the stent is fully expanded against the artery wall, which can significantly reduce the risks of stent thrombosis and restenosis, thereby improving the overall success of the procedure17. It is essential that healthcare providers are aware of such complications to take the necessary precautions to minimise risks during and after the procedure18. In addition, the analysis in the present study suggests that the Medina classification was also a significant factor affecting cardiac death; this emphasises the significance of lesion complexity on patient outcomes. Certain configurations, such as true bifurcation lesions, have been associated with poor prognoses post-PCI19.
It is essential to highlight the continuous sinusoidal design of the Resolute stents, which have shown greater resistance to longitudinal deformation when compared to other stents such as OMEGA and PROMUS Element (both Boston Scientific), or Endeavor and Driver (both Medtronic) and similar resistance to the TAXUS Liberté (Boston Scientific), MULTI-LINK 8 XIENCE Prime, Vision, and XIENCE V (all Abbott Vascular)20. This technological advantage may contribute to the favourable outcomes observed in the present study, as evidenced by the high success rates in device delivery that were reported in trials such as the RESOLUTE All-Comers and the Real-World Endeavor Resolute Versus XIENCE V Drug-Eluting Stent Study in Twente (TWENTE)2122.
In contrast to previous studies such as SYNTAX9 and EXCEL14, which reported higher mortality rates in diabetic patients undergoing PCI, our study found comparable mortality outcomes between diabetic and non-diabetic patients treated with the Resolute ZES. This discrepancy may be attributed to several factors. Firstly, our cohort benefited from the use of second-generation DES, which have demonstrated improved safety profiles and reduced rates of restenosis and stent thrombosis compared to older devices. Additionally, the high-volume experience of our centre in performing LM PCI, combined with the implementation of advanced procedural techniques, including post-dilatation and careful patient selection, may have contributed to these favourable outcomes. Moreover, our study population may have been more carefully selected, with comprehensive management of comorbid conditions, thus mitigating the typical adverse effects associated with diabetes in other studies. These factors highlight the importance of not only the technology used but also the clinical expertise and procedural strategies in achieving successful outcomes in high-risk populations.
The present study was conducted at a high-volume, single-centre tertiary care institution with extensive experience in left main PCI, which may also have contributed to the observed low mortality rates. However, the success achieved in this cohort is largely attributed to the use of standardised and advanced interventional techniques, such as the deployment of the Resolute ZES, routine post-dilatation, and the careful selection of stenting techniques based on lesion complexity. These practices, supported by intravascular imaging and contemporary guidelines, are transferable to other centres. Although high-volume centres with experienced operators are often associated with better outcomes, we believe that adherence to best practices and the use of appropriate technology can enable similar results in centres with less experience in LM PCI.
Training programmes, collaboration with high-volume centres, and the dissemination of procedural protocols are crucial steps to help ensure the reproducibility of these outcomes across a wider range of institutions. Additionally, future multicentre registries or randomised trials could help validate these findings in more diverse clinical settings.
This study, while offering valuable insights into the outcomes of left main PCI using the Resolute ZES, has several limitations that must be acknowledged. First, of the 115 patients excluded from the study due to lack of follow-up information, 58.26% (67 patients) were diabetic. The exclusion of these patients may introduce bias, as the prevalence of diabetes in this group is notable and could affect the overall study outcomes, especially considering that diabetes is a significant risk factor for cardiovascular events. Although the overall sample size remains robust, the exclusion of these patients may have introduced a degree of selection bias, potentially underestimating or overestimating mortality and other event rates.
Second, the data were collected retrospectively from a single centre, which limits the generalisability of the findings. While our centre is highly experienced in LM PCI, centres with less experience or lower procedural volumes may not replicate similar outcomes.
Additionally, follow-up data were primarily obtained through phone calls, which may lack the granularity needed for detailed assessments of postprocedural complications or quality-of-life metrics. The inability to capture MACE, including non-fatal MI or stroke, also limits our ability to draw comprehensive conclusions regarding the long-term safety profile of the stents.
The absence of SYNTAX score collection is also one of the limitations of this study; collecting the score could have provided additional insights into the complexity of coronary artery disease and further enhanced the model-building process.
Finally, the rarity of cardiac death events limits the statistical power of the Cox regression models, particularly when assessing the impact of specific procedural techniques or lesion characteristics on patient outcomes. Future studies with larger patient cohorts and prospective designs are needed to validate these findings.
This study supports the safety and effectiveness of the Resolute ZES in a real-world setting, with comparable outcomes between diabetic and non-diabetic patients. It also identifies key factors that could guide clinical decisions and highlights the potential for further refinement in managing patients with complex coronary artery disease. However, further prospective studies with more comprehensive data collection are necessary to validate these results and refine the selection criteria for optimal revascularisation strategies in diabetic patients.
Conclusions
The findings of this study support the effectiveness of the Resolute ZES in patients undergoing left main PCI, demonstrating comparable outcomes in patients with and without diabetes. Given the complexities associated with managing LMCA disease, further prospective studies are necessary to validate these findings and explore the comparative effectiveness of PCI versus CABG in diverse patient populations. Individualised treatment approaches, considering patient-specific factors and lesion characteristics, remain essential for optimising outcomes in this high-risk group. Overall, this study highlights the importance of ongoing assessment of procedural techniques and outcomes in enhancing patient care in the context of coronary artery disease management.
Impact on daily practice
This study demonstrates that the Resolute zotarolimus-eluting stent (ZES) has comparable efficacy in diabetic and non-diabetic patients undergoing left main percutaneous coronary intervention (PCI). The absence of significant differences in mortality rates between the two groups underscores the viability of the Resolute ZES as a treatment option for diabetic individuals with coronary artery disease. Notably, the identified predictors of cardiac death, including age and renal function, emphasise the importance of personalised management strategies in optimising outcomes. This underscores the relevance of considering individual patient characteristics and procedural techniques in clinical decision-making for left main PCI, particularly in populations with diabetes.
Acknowledgements
The authors would like to thank the National Heart Foundation of Bangladesh for the research grant and BioQuest Solutions Pvt. Ltd. for editorial support.
Funding
The study was funded by the National Heart Foundation of Bangladesh. The funding agency had no role in the study design, execution, analysis, or interpretation of results.
Conflict of interest statement
The authors have no conflicts of interest to declare.