Introduction
Current expert opinion supports the use of low-speed rotational atherectomy (LS-RA) as an optimal technique1 because slower burr speeds reduced platelet aggregation and heat injury associated with RA in previous in vitro experiments2,3. However, a previous in vivo trial did not show benefits of LS-RA over high-speed RA (HS-RA) in terms of prevention of slow flow following RA4. In contrast, another effect of LS-RA may be that it ablates more plaque than does HS-RA because the burr axis vibrates to a greater extent while the burr rotates at slower speeds, like the precessional movement of a spinning top. However, this additional ablation effect of LS-RA following HS-RA has not been completely elucidated. We hypothesised that LS-RA following HS-RA can achieve more acute gain by ablating more plaque than does HS-RA alone. To test this hypothesis, we conducted intravascular ultrasound (IVUS) analysis before and after LS-RA following HS-RA.
Methods
Of the 66 patients who underwent RA between January 2015 and July 2016, we examined 26 patients with 27 de novo severely calcified lesions who underwent LS-RA following HS-RA. Demographic, angiographic, and procedural data were collected from a prospectively entered dedicated database. This study was approved by the research review board of Ogaki Municipal Hospital and conducted according to the Declaration of Helsinki.
RotaLink™ Plus (Boston Scientific, Marlborough, MA, USA) was used in all patients. As wires, RotaWire™ floppy or RotaWire™ Extra Support (Boston Scientific) were used. The burr was advanced with a gradual push-forward/pull-back motion over short individual runs (<30 s) while attempting to avoid extreme deceleration. The rotational speed was set at 200,000 rpm at platform in the HS-RA protocol and at 115,000 rpm at platform in the LS-RA protocol. RA in both protocols was continued until the rotational speed deceleration of <2,000 rpm was achieved. Only one burr was used for each lesion. The study protocol was as follows: (1) HS-RA; (2) IVUS (before LS-RA); (3) LS-RA; and (4) IVUS (after LS-RA). Selection of wires and percutaneous coronary intervention (PCI) strategies, except for RA, was at the discretion of the treating physician.
The objective of this study was to compare the minimal lumen area (MLA) between IVUS findings before and after LS-RA. To obtain the corresponding MLA images before and after LS-RA, distances from at least two landmarks, such as side branches and calcifications, were used as references. Two experienced clinical engineers (CE) blinded to the study object independently measured IVUS. Any discrepancies between the two CEs were resolved by consensus.
MLA before and after LS-RA was compared using a paired t-test. All statistical analyses were performed using JMP software version 13.1 (SAS Institute Inc., Cary, NC, USA). p<0.05 was considered statistically significant.
Results
Baseline characteristics are summarised in Supplementary Table 1. Regarding PCI indication, most were cases of stable angina pectoris; however, there were four cases of non-ST-elevation acute coronary syndrome. The target lesions were the right coronary artery in 9, and the left anterior descending artery in 14 patients. Before RA, an IVUS catheter could be passed through the lesion in 15 cases, and predilatation was attempted in 9 lesions. All but 2 lesions were ablated using RotaWire floppy; the burr size was 1.5 mm in 11, 1.75 mm in 11, and 2 mm in 5 lesions. While performing LS-RA, a rotational speed deceleration of >3,000 rpm was obtained even after after HS-RA which ablated until a rotational speed deceleration of <2,000 rpm.
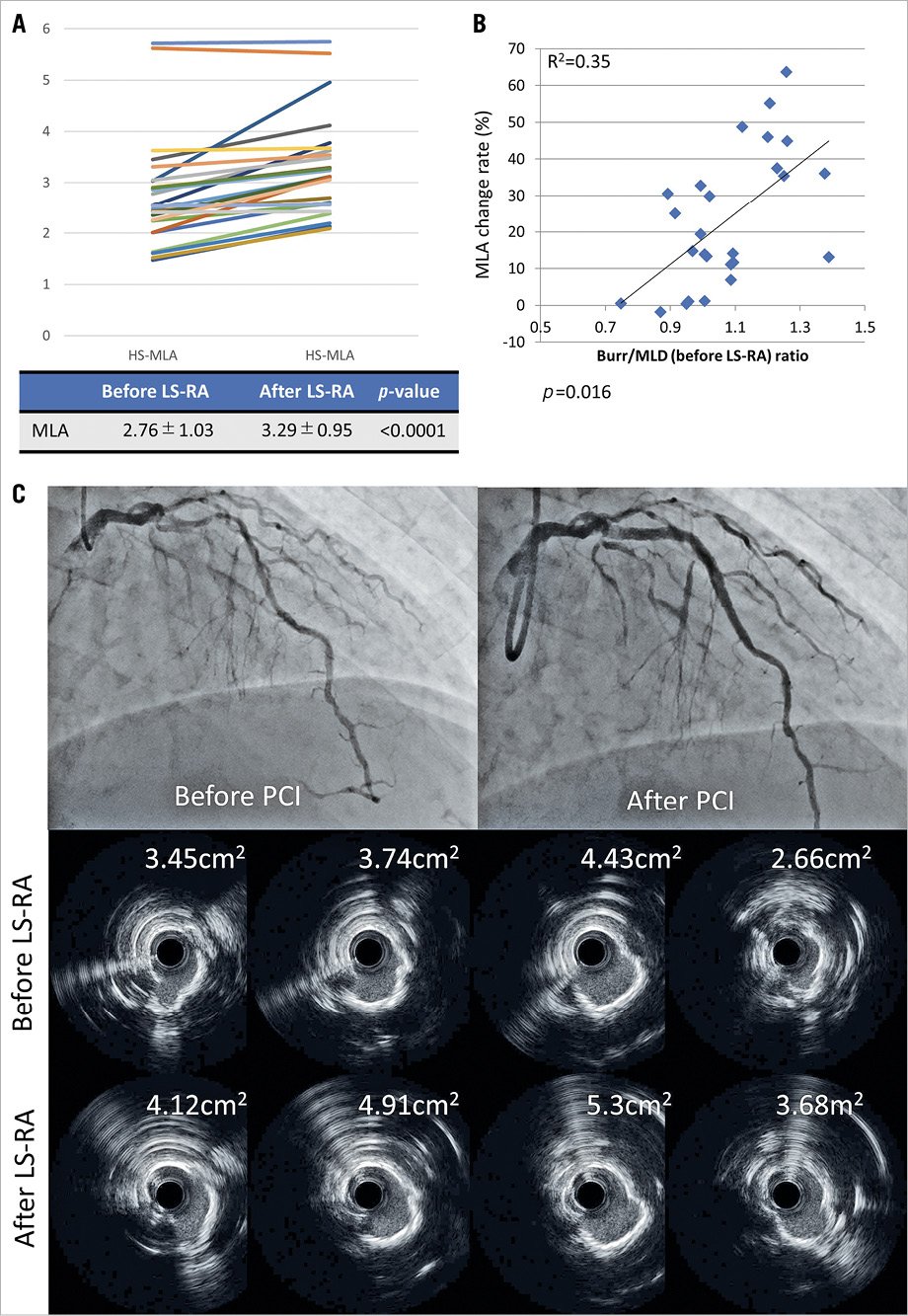
Figure 1. Changes in minimum lumen area. (A) The change in MLA before and after low-speed rotational atherectomy (LS-RA) in each patient. (B) The relationship between minimal lumen area (MLA) change and burr-to-minimal lumen diameter (MLD) ratio. (C) Representative case. At each point, larger lumen areas were obtained following additional LS-RA.
MLA after LS-RA was significantly larger than that before LS-RA (2.76 ± 1.03 mm2 vs 3.29 ± 0.95 mm2; p<.0001; Figure 1A). The larger the burr-to-minimum lumen diameter (before LS-RA) ratio, the greater the change in the MLA before and after LS-RA (Figure 1B). A representative case is presented in Figure 1C. Slow flow occurred in 4 lesions after LS-RA, which recovered immediately after intracoronary nitroprusside injection. Final Thrombolysis in Myocardial Infarction flow grade 3 could not be attained in 3 lesions, in which slow flow developed before LS-RA. No major vessel perforation was observed.
Discussion
In this study, we demonstrated that a significantly larger acute gain was achieved with additional LS-RA following HS-RA. Although we did not demonstrate the relationship between adding LS-RA and optimal stent expansion compared with standard RA, the mean additional ablation of 330 μm (calculated from MLA) may be helpful, considering that a previous study demonstrated the thresholds of calcium thickness of 450 μm as the predictor of calcium crack5. Because the burr-to-artery ratio is the significant determinant of slow flow4, performing additional LS-RA following HS-RA, rather than increasing the burr size, may be better to reduce complications and cost. The gyro effect may explain these results; the gyro effect is the principle that a burr rotating at a high-speed with a high moment of inertia proportional to the rotational speed produces less degree of change in the rotation axis direction6.
Limitations
This is a pilot study with a retrospective nature. Eventually, this hypothesis needs to be tested in a prospective trial with proper randomisation. The wire bias may have influenced the ablation effect of LS-RA, suggesting that this technique may not be applied to all lesions, for example, eccentric calcified lesions. The burr speed used in the HS-RA protocol was in excess of the expert consensus speed1, which might compromise the MLA increase or lead to more complications than might occur in the recommended high-speed protocol. Furthermore, the optimal speed for LS-RA has not yet been elucidated. Finally, although HS-RA was continued until the rotational speed deceleration of <2000 rpm was achieved, the increase in MLA may simply have been gained by additional ablation rather than the effect of LS-RA. Furthermore, image analysis was not performed by a core laboratory.
Conclusions
Additional LS-RA following HS-RA ablated more plaque volume and achieved significantly larger MLA than HS-RA alone.
Impact on daily practiceAdditional LS-RA following HS-RA could achieve more acute gain by ablating more plaque than HS-RA alone, and might contribute to decreased incidence of slow flow and medical costs compared to increasing the burr size. |
Funding
The Department of Cardiology, Nagoya University Graduate School of Medicine received research grant from Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Pfizer Japan Inc., and Teijin Pharma Ltd.
Conflict of interest statement
H. Ishii received lecture fees from Astellas Pharma Inc., Bayer Pharmaceutical Co., Ltd., Daiichi-Sankyo Pharma Inc., and MSD K. K. T. Murohara received lecture fees from Bayer Yakuhin., Ltd., Daiichi-Sankyo Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd. The other authors have no conflicts of interest to declare.

