Introduction
The field of interventional cardiology has evolved significantly in the four decades since the first percutaneous coronary angioplasty. Plain old balloon angioplasty has progressed to more refined technologies such as bare metal stents and drug-eluting stents (DES). Despite the significant decrease in the risk of restenosis associated with metal stents as compared to balloon angioplasty, these devices may alter vasomotor function in the stented segment1.
Some studies have documented the preservation of normal vasomotor function proximal and distal to the stent2. However, others have identified endothelial dysfunction in these regions that could be attributed to the rigidity imposed by the stent1. This rigidity makes the stented segment behave as a stiff and non-compliant vessel. The altered distensibility may compromise the vasodilatory effect of endogenously produced nitric oxide as well as its protective effect3. Metallic implantation for continuous vessel support not only hinders beneficial vessel restructuring and the reinstatement of vascular physiology but also complicates future attempts to revascularise the targeted vessels4.
Innovative bioresorbable vascular scaffold (BVS) technology has eliminated the stiffness issue because the implanted device degrades over time after implantation, thereby restoring the natural properties of the vascular wall4. The first-generation BVS were developed with a larger crossing profile, thicker struts, a lack of radiopacity, and limitations related to expansion. In addition to these structural and mechanical limitations, there were some safety concerns, especially the increased risk of very late scaffold thrombosis5.
Hence, there is a need for next-generation BVS with an improved design, especially a reduced strut thickness and lower device profile. These advances may lead to optimal endothelialisation and lower scaffold thrombosis rates5.
Malapposition and underexpansion are the two most frequent technical problems that result in adverse outcomes following a percutaneous coronary intervention (PCI) procedure. To minimise these adverse outcomes, the “predilatation, vessel sizing, and post-dilatation” (PSP) technique has been introduced6. The next-generation MeRes100 bioresorbable scaffold (BRS; Meril Life Sciences) is available with a complete PSP tool kit containing two BioMime Lineage non-compliant (NC) balloons (also Meril Life Sciences; one balloon is of the same diameter as the scaffold and the other is 0.25 mm or 0.50 mm wider) along with the scaffold (Supplementary Table 1).
Methods
Study design
This was a retrospective, single-centre study that included 86 patients who were treated at ACE Heart and Vascular Institute, Mohali, India, from 8 February 2021 to 5 October 2023. All patients were implanted with MeRes100 BRS and followed for up to 12 months. A total of 73 patients completed the 6-month follow-up visit, and 55 patients completed the 12-month follow-up visit. Only one patient died during hospitalisation (cardiac death, not related to the device).
Data were collected retrospectively from electronic medical records by trained study personnel. Variables of interest included demographic characteristics, medical history, lesion characteristics, procedural characteristics, and treatment outcomes.
The study protocol was approved by the local institutional ethics committee, and the study was performed in compliance with the Declaration of Helsinki.
The study population included patients implanted with MeRes100 BRS for primary and recurrent in-stent restenosis, diffuse disease, coronary artery graft failure, a bifurcation lesion (0,1,1), and hybrid PCI with left main coronary artery bifurcation. This is a retrospective data collection study; hence there were no formal exclusion criteria.
The clinical endpoint of the study was the incidence of major adverse cardiac events (MACE) – a composite of cardiac death, any myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (ID-TLR) – and scaffold thrombosis.
Definitions of endpoints
Procedural success: the successful deployment of at least one study device at the intended target lesion, successful withdrawal of the delivery system, final in-scaffold diameter stenosis <30% (by quantitative coronary angiography [QCA]), and no in-hospital (maximum 7 days) target lesion failure.
Cardiac death: any death due to proximate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, all procedure-related deaths including those related to concomitant treatment78.
Periprocedural MI (within 48 hours of a revascularisation procedure)78: the criteria for periprocedural MI in three patient populations are given below.
1) Patients with stable coronary artery disease (CAD), or silent ischaemia, or acute coronary syndromes (ACS) with at least two baseline troponin values which remain below the upper limit of normal (ULN), or ACS with troponin and/or creatine kinase-myoglobin band (CK-MB) levels that have been raised but all return to
2) Patients with stable CAD and a raised baseline CK-MB, or ACS, in whom at least two baseline troponin and CK-MB values have been drawn and the most recent troponin and CK-MB values are lower than the previous measures by >25%: an absolute incremental CK-MB rise within 48 hours of the procedure from the previous most recent CK-MB level by >5xULN for post-PCI or >10xULN for post-CABG.
3) Patients with raised baseline CK-MB in whom the biomarker levels have not been revealed to be stable or falling (either because only one CK-MB is measured, or the most recent CK-MB measure in a series is either still increasing or has not decreased by >25% from the most recent measure): the CK-MB rises within 48 hours of the procedure by an absolute increment from the previous most recent CK-MB level of >5xULN for post-PCI or >10xULN for post-CABG.
In addition, the following must also be present:
1) new ST-segment elevation or depression, and
2) signs consistent with a clinically relevant MI, such as new-onset or worsening heart failure or sustained hypotension.
Spontaneous MI (before or >48 hours after any coronary revascularisation procedure): troponin >ULN or CK-MB >ULN, plus one or more of the following must also be present: symptoms of ischaemia; electrocardiogram (ECG) changes indicative of new ischaemia – new ST-T changes or new left bundle branch block, development of pathological Q waves78; imaging evidence of a new loss of viable myocardium or a new regional wall motion abnormality.
Target vessel-related MI: MIs which cannot be definitively adjudicated to a non-target vessel are considered target vessel-related MIs78.
Non-target vessel-related MI: all MIs which are not target vessel-related MIs78.
Target lesion revascularisation (TLR): any repeat percutaneous intervention of the target lesion, or bypass surgery of the target vessel performed for restenosis, or other complications of the target lesion78.
Ischaemia-driven revascularisation: a revascularisation is considered ischaemia driven if it is associated with any of the following: positive functional ischaemia study including positive fractional flow reserve or ischaemic symptoms and angiographic diameter stenosis ≥50% by QCA or angiographic diameter stenosis ≥70% by QCA without angina or a positive functional study78.
Any revascularisation: any target vessel revascularisation (TVR) or non-TVR, whether by PCI or CABG78.
Scaffold thrombosis: scaffold thrombosis is defined as the presence of a thrombus that originates in the scaffold or in the segment 5 mm proximal or distal to the scaffold, with at least one of the following criteria within 48 hours of the procedure: (1) acute onset of ischaemic symptoms at rest; (2) new ischaemic ECG changes that suggest acute ischaemia; (3) typical rise and fall in cardiac biomarkers; (4) non-occlusive thrombosis (a spherical, ovoid, or irregular non-calcified filling defect or lucency surrounded by contrast material on 3 sides or within a coronary stenosis seen in multiple projections, or persistence of contrast material within the lumen), or a visible embolisation of intraluminal material downstream; (5) occlusive thrombus (Thrombolysis in Myocardial Infarction [TIMI] 0 or 1) intrastent/scaffold or proximal to a stent/scaffold up to the most adjacent proximal side branch or main branch (if originating from the side branch)78.
Severity of calcification: classified as none or mild, moderate (density seen only with cardiac motion before contrast medium injection on one side of the arterial wall), and severe (radiopacity seen without cardiac motion before contrast medium injection generally on both sides of the arterial wall)9. The definitions of the types of lesions are provided in Supplementary Appendix 1.
Device description
The MeRes100 BRS is a next-generation balloon-expandable, sirolimus-eluting BRS. It is composed of a poly L-lactide (PLLA) polymer backbone with a strut thickness of 100 μm, which is coated by a thin layer of mixture containing the polymer poly-D, L-lactide (PDLLA) and the antiproliferative drug sirolimus in the ratio of 1:1. The biodegradable polymer − PDLLA − acts as a drug reservoir and releases the sirolimus drug (drug dose: 1.25 μg/mm2) in a controlled manner. The PLLA and PDLLA biodegradable polymers degrade in the body via the hydrolysis of ester bonds and ultimately result in the formation of CO2 and H2O, which are eliminated from the body. This enables the scaffold to fully disappear from the treatment site within 24 to 36 months of implantation. Its hybrid cell design includes closed cells at the edges and open cells in the mid-segment, which allow optimal vessel wall conformability. The presence of three pairs of platinum radiopaque markers that are positioned 120° apart from each other at either end of the scaffold facilitates angiographic placement. Furthermore, the design includes variations in strut width which enable the scaffold to retain its high radial strength without affecting its flexibility, even with low strut thickness. The MeRes100 BRS is available in diameters ranging from 2.25 mm to 4.50 mm and lengths ranging from 8 mm to 40 mm (Figure 1).
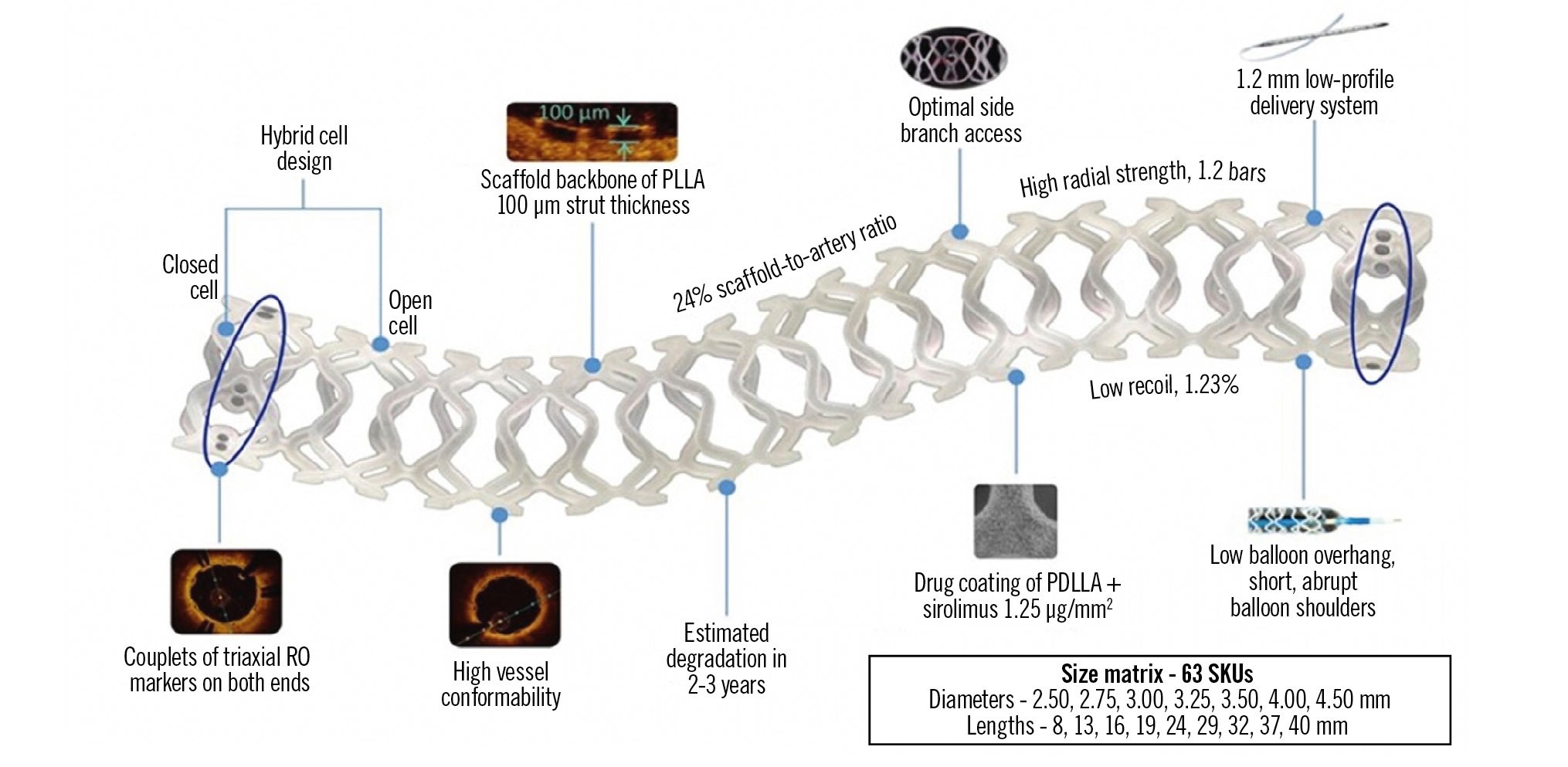
Figure 1. Design and features of the MeRes100 bioresorbable scaffold. PDLLA: poly-D, L-lactide; PLLA: poly L-lactide; RO: radiopaque
MeRes100 BRS implantation technique
The MeRes100 BRS is available with a complete PSP tool kit, which includes Lineage NC balloons in lengths of 10 mm and 15 mm. The 10 mm balloon is designed to be compatible with MeRes100 BRS lengths of 16 mm, 19 mm, and 24 mm, while the 15 mm Lineage NC balloon is intended for use with MeRes100 BRS lengths of 29 mm, 32 mm, and 40 mm (Supplementary Table 1).
During the MeRes100 BRS implantation procedure, predilatation is a mandatory step that should be performed with a balloon that is either 0.5 mm smaller in diameter or at least 1:1 sized with the reference vessel diameter (RVD). It is advised to use cutting/scoring balloons or atherectomy for calcified lesions or those that do not fully predilate. Achieving full balloon expansion is essential for scaffold implantation. The predilatation balloon expansion should be maintained up to its nominal pressure. In order to achieve proper vessel-device sizing, optimal deployment, and apposition of scaffold struts, PCI procedures under optical coherence tomography (OCT) or intravascular ultrasound (IVUS) guidance are recommended56. Intravascular imaging should be strongly considered if the visual RVD is <3 mm or a 2.5 mm BRS is planned. Implantation of the scaffold should not be performed if the RVD is <2.5 mm. Following scaffold deployment, it is necessary to perform post-dilatation at high pressure (≥18 atmospheres), using either an optimal balloon size or an NC balloon that is 0.5 mm larger, to achieve a residual diameter stenosis of ≤10%. However, the post-dilatation balloon should never be more than 0.5 mm larger than the scaffold’s nominal diameter. Here, we present the results of a single-centre study of MeRes100 in real-life clinical practice.
Statistical analysis
Descriptive statistical parameters were calculated to analyse the study findings, such as mean and standard deviation or absolute count and percentage for numerical values, and absolute count and percentage for categorical values.
Results
The patient demographics were diverse and well documented. The mean age of the study population was 61.86±11.25 years. A total of 63 of 86 patients (73.25%) were male. Overall, 37 patients were diagnosed with single-vessel disease, 31 with double-vessel disease, and 18 were found to have triple-vessel disease. Twelve patients (14%) were smokers. The complete baseline characteristics are provided in Table 1.
The most common comorbidities included hypertension (72.09%), diabetes (43.03%), and unstable angina (56.97%) (Central illustration). In all, 31 patients (36.04%) had a history of prior PCI/CABG. Full characterisation of patient medical history is given in Table 1. A total of 140 stenotic lesions were treated in this study. The majority of these were de novo lesions, accounting for 90% (n=126). In-stent lesions accounted for 10% (n=14). Lesion calcification, a substantial factor influencing the success of stent implantation, was found by IVUS in 26 patients. Lesion locations are detailed in Table 2. The classification of the lesions according to ACC/AHA definitions is shown in Figure 2. Additionally, the study population featured a wide variety of lesion locations, as seen in real-life clinical practice. Full lesion characteristics can be found in Table 2.
Table 1. Baseline demographic characteristics and medical history of the patients.
| Patient characteristics | N=86 |
|---|---|
| Age, years | 61.86±11.25 |
| Female | 23 (26.74) |
| Male | 63 (73.25) |
| BMI, kg/m2 | 26.43±4.22 (n=84) |
| Heart rate, beats/min | 81.24±16.28 (n=74) |
| Systolic BP, mmHg | 135.15±21.08 (n=75) |
| Diastolic BP, mmHg | 80.21±12.09 (n=75) |
| Serum creatinine, mg/dl | 1.11±0.69 (n=63) |
| Single-vessel disease | 37 (43.02) |
| Double-vessel disease | 31 (36.05) |
| Triple-vessel disease | 18 (20.93) |
| Comorbidities | |
| Hypertension | 62 (72.09) |
| Diabetes | 37 (43.03) |
| Prior PCI/CABG | 31 (36.04) |
| Prior rhythm abnormalities | 0 (0) |
| CKD (eGFR <60 ml/min/1.73 m2) | 20 (23.25) |
| Prior CVA/stroke | 2 (2.32) |
| MI and unstable angina status | |
| CCS | 10 (11.62) |
| STEMI | 15 (17.44) |
| NSTEMI | 12 (13.95) |
| Unstable angina | 49 (56.97) |
| Conduction abnormality | |
| LBBB | 3 (3.49) |
| RBBB | 1 (1.16) |
| Values are presented as n (%) or mean±SD, based on N=86 unless otherwise stated. BMI: body mass index; BP: blood pressure; CABG: coronary artery bypass graft; CCS: chronic coronary syndrome; CKD: chronic kidney disease; CVA: cerebrovascular accident; eGFR: estimated glomerular filtration rate; LBBB: left bundle branch block; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; RBBB: right bundle branch block; SD: standard deviation; STEMI: ST-elevation myocardial infarction | |
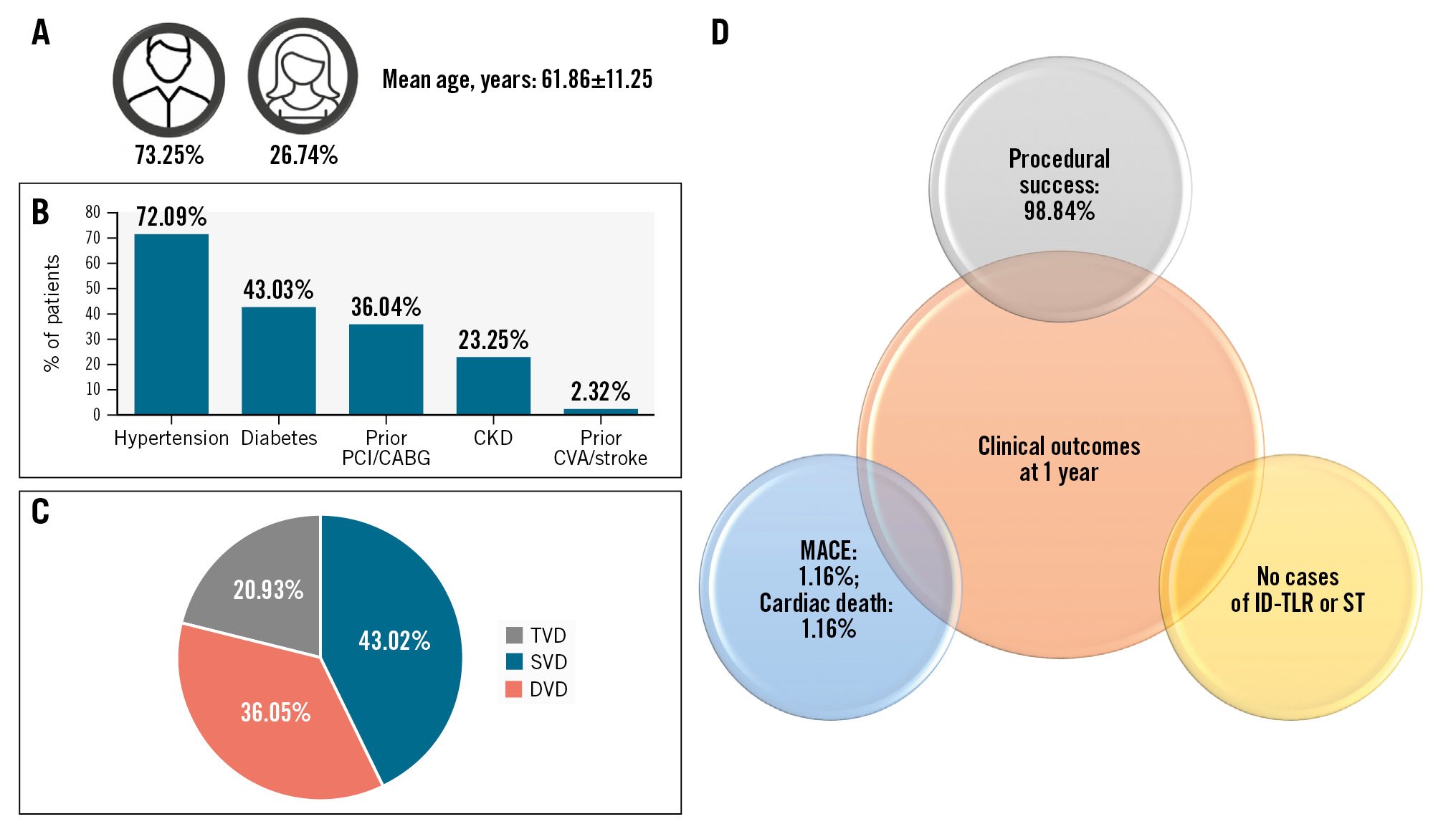
Central illustration. Real-world experience with MeRes100 BRS. MeRes100 BRS appeared to be a safe and effective strategy for complex de novo and in-stent restenotic coronary lesions. A) Patient demographics; (B) comorbidities; (C) patterns of coronary artery disease; (D) 1-year clinical outcomes. BRS: bioresorbable scaffold; DVD: double-vessel disease; ID-TLR: ischaemia-driven target lesion revascularisation; MACE: major adverse cardiac events; ST: stent thrombosis; SVD: single-vessel disease; TVD: triple-vessel disease
Table 2. Lesion distribution and characteristics in 86 patients treated with the next-generation sirolimus-eluting bioresorbable vascular scaffold system.
| Lesion characteristics | Prevalence |
|---|---|
| Total no. of lesions | 140 |
| Lesion location | |
| LAD/D1 | 84 lesions, 50 patients |
| RCA/PDA/PLV | 40 lesions, 26 patients |
| LCx/OM/Ramus | 16 lesions, 10 patients |
| Type of lesion | |
| De novo | 126 (90.00) |
| In-stent | 14 (10.00) |
| Total no. of lesions treated with MeRes100 BRS | 104 |
| Lesion length, mm | 31.5±18.3 |
| Reference vessel diameter, mm | 3.13±0.38 |
| Diameter stenosis, % | 82.2±12.4 (n=100) |
| Minimal luminal diameter, mm | 0.56±0.25 |
| LVEF, % | 56.71±9.73 (n=87) |
| Lesion calcification: fluorescence+IVUS | 26 (18.50) |
| Mild | 8 (30.76) |
| Moderate | 10 (38.46) |
| Severe | 8 (30.76) |
| Values are presented as n (%) or mean±SD, based on N=140 unless otherwise stated. BRS: bioresorbable scaffold; D1: first diagonal branch; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCx: left circumflex artery; LVEF: left ventricular ejection fraction; OM: obtuse marginal branch; PDA: patent ductus arteriosus; PLV: posterior left ventricular artery; Ramus: ramus intermedius coronary artery; RCA: right coronary artery; SD: standard deviation | |
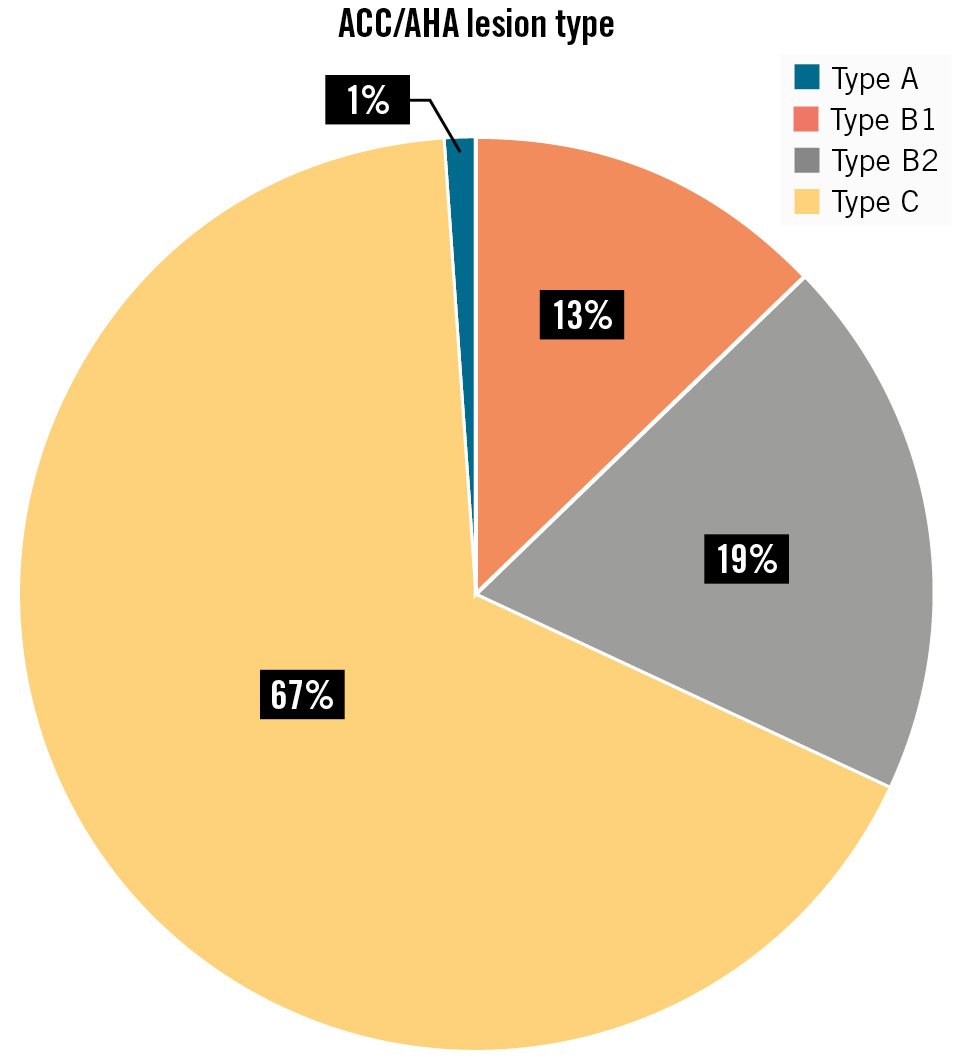
Figure 2. Lesion type according to ACC/AHA.
Procedural characteristics
A total of 104 MeRes100 bioresorbable scaffold implants were performed during the study (0.74 implants per lesion on average). Metal drug-eluting stents were also used in hybrid PCI, which involves overlapping BRS and DES in the same vessel, and this was carried out in 33 cases with multiple lesions. All procedures were performed by the same operator.
In addition to the default NC balloon included in the MeRes100 BRS PSP tool kit, a scoring balloon was used in 35 cases, a drug-eluting balloon in 2 cases, and an ultrahigh-pressure balloon was used in 3 cases. Intravascular lithotripsy was applied in 4 cases for bed preparation, and rotablation was applied in another 4 cases.
Radial right access was used in most cases (86.05%). The femoral right access (11.63%) and femoral left access (1.16%) were also used. IVUS was used intraoperatively in 26 cases (18.5%). The diameters and lengths of the implanted stents varied widely. Stent placement included ostial (11.54%), proximal (27.88%), mid (36.54%), and distal (24.04%). All procedural characteristics are summarised in Supplementary Table 2.
In the course of this study, predilatation and post-dilatation were applied to all lesions. The success rate of the index procedure was 98.84%, as the delivery of the scaffold/stent to the target lesion and appropriate deployment were reported in all but two cases. Additionally, the delivery system was flawlessly removed after scaffold/stent release in all cases. All cases except for one achieved <30% residual diameter stenosis of all treated lesions by visual inspection or QCA. The outcomes are described in detail in Table 3. The TIMI flow before and after the procedure are compared in Figure 3.
Follow-up data were obtained from outpatient visits and follow-up phone calls. The average duration of hospital stay post-procedure was 2.5 days, with a standard deviation of 1.68 days. The hospital records demonstrated beneficial clinical outcomes, with a 1.16% (n=1) in-hospital mortality rate and no cases of ID-TLR or scaffold thrombosis at follow-up. The MACE rate was 1.16%.
The one patient who died had triple-vessel disease and had undergone PCI with one MeRes100 BRS to the mid-proximal left anterior descending artery and two DES to the distal left circumflex artery. Procedural success was achieved with the successful delivery of the scaffold and stents, followed by appropriate deployment and successful removal of the delivery system. A postprocedural TIMI 3 flow gradient was achieved. The patient died in hospital (before discharge) with target vessel MI.
Table 3. Procedural outcomes.
| Device success – at index procedure | |
|---|---|
| Successful delivery of the scaffold/stent to the target site | |
| Yes | 104 (100) |
| No | 0 (0) |
| Appropriate deployment | |
| Yes | 102 (98.08) |
| No | 2 (1.92) |
| Successful removal of the delivery system after stent/scaffold release | |
| Yes | 86 (100) |
| No | 0 (0) |
| At discharge | |
| Length of index hospital stay, days | 2.50±1.68 (n=86) |
| Procedural success – at discharge | |
| Procedural success* | 86 (100) |
| Achieved <30% residual diameter stenosis of all treated lesions by visual inspection or QCA | 85 (98.84) |
| The values are given as an absolute number and percentage, or as mean±SD. *Overlap miss was confirmed on IVUS in 1 case (1.16%) at follow-up. Scaffold overexpansion, leading to microaneurysm formation on follow-up was reported in 1 case (1.16%). IVUS: intravascular ultrasound; QCA: quantitative coronary angiography; SD: standard deviation | |
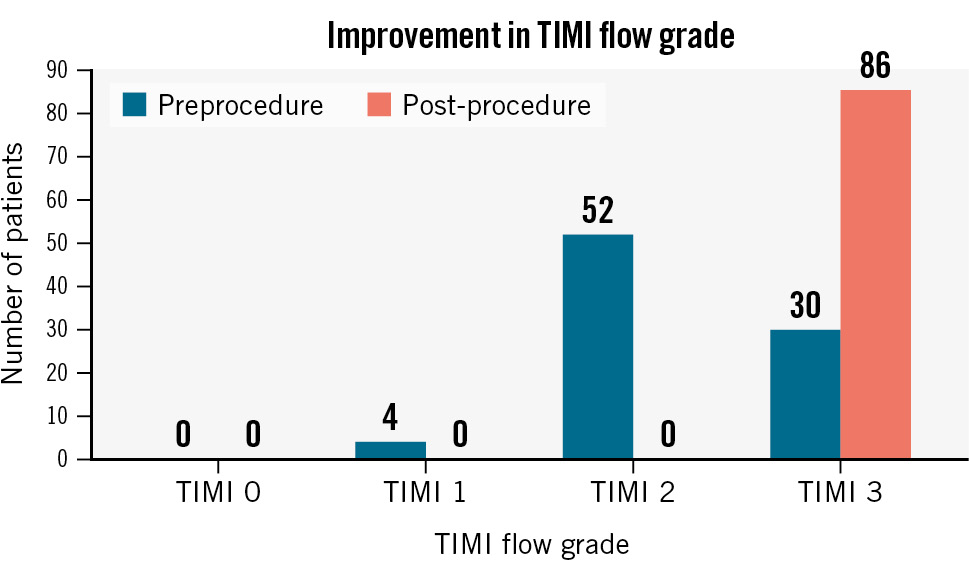
Figure 3. TIMI flow before and after implantation of the MeRes100 bioresorbable scaffold. TIMI: Thrombolysis in Myocardial Infarction
Discussion
The MeRes100 BRS system demonstrated sustained efficacy and safety with maintained lumen patency and no very late scaffold thrombosis in the MeRes-1 study up to 3-year follow-up10. Current study findings further confirm the safety and efficacy of MeRes100 BRS with no MACE, cardiac death, MI, ID-TLR, or scaffold thrombosis in an all-comer patient population undergoing PCI with MeRes100 BRS for diverse indications.
A bioresorbable vascular scaffold offers the advantage of resorption after the artery is restored to its natural state. This implies the potential for freedom from cardiac events, the option to discontinue antiplatelet therapy if necessary and facilitate the possibility of bypass grafting of these vessels in the future8. The first generation of the BRS had several shortcomings. It was a bulky device with a large profile that had some unique technical features, making it challenging to deliver and implant. Although it was effective, the primary concern with the first-generation BRS was its safety11. The MeRes100 has numerous beneficial enhancements to ensure user-friendliness, such as a unique hybrid cell design with closed cells at the edges and open cells along the length. It also has a low strut thickness of 100 μm, excellent radiopacity with triaxial platinum radiopaque markers placed circumferentially 120º apart at both ends of the scaffold, and a high radial strength of 1.2 bars. Additionally, the scaffold is highly deliverable and flexible, and it is available in a range of scaffold lengths and diameters.
Based on observations of PCI procedures in the study population, the use of a BRS system requires certain important considerations. The vessel bed must be prepared with appropriately sized balloons (semicompliant, non-compliant, ultrahigh-pressure angioplasty balloons) or atherectomy prior to the procedure. To ensure precision and control in manoeuvring, distal tracking of the device can be aided by using a buddy-wire guide extension catheter. Deployment of the device to nominal pressures should take at least 1 minute. Post-dilatation with MeRes100 should not exceed a pressure of 24 atmospheres with NC balloons to prevent device damage11. Additionally, a meta-analysis of data from five ABSORB prospective studies found that an optimal implantation technique, including careful post-dilatation, can improve clinical outcomes after BVS implantation12.
For hybrid PCI, careful case selection is critical, with evidence suggesting a benign interaction between the metal stent and the scaffold1314. On the other hand, findings from the prospective GABI-R registry suggest that the clinical outcomes of hybrid PCI can be similar to those, or worse, with BVS implantation without a metal stent. The authors of the GABI-R registry suggested that comorbid patients treated with hybrid stenting had less successful outcomes due to the higher complexity of the lesions in these patients15.
Adopting sound implantation techniques, preferably in combination with imaging, has been associated with remarkably low event rates16. We used IVUS in 26 cases in this study and confirmed this conclusion. To ensure successful treatment, it is compulsory to maintain close and regular clinical follow-ups after the procedure as well. Additionally, follow-up angiography timing may vary, depending upon the clinical parameters and stress test results, and it could be scheduled in the third year.
When compared to other European Conformity (CE)-marked BRS, the MeRes100 BRS has lower rates of MACE and stent thrombosis. This may be due to its thinner-strut design (100 μm), which sets it apart from other CE-marked scaffolds like DESolve (Elixir Medical; 150 μm), Magmaris (Biotronik; 150 μm), and Absorb (Abbott; 156 μm). It was observed that BRS with thicker struts had higher target lesion revascularisation rates, with DESolve at 7.4%, Magmaris at 5.9%, and Absorb at 7.4%, in comparison to MeRes100 at 1.61% at 2-year follow-up1718192021. It is worth noting that, while these recommendations and insights provide valuable guidance for immediate and short-term management, the long-term implications of BRS systems necessitate additional studies. A comparison of technologies of the current-generation thinner-strut BRS (Absorb BVS, DESolve Nx/Cx, FORTITUDE [Amaranth Medical], Mirage [Manli Cardiology], Firesorb [MicroPort Medical], and Fantom [REVA Medical]) has been tabulated in Table 42223.
Table 4. Comparison of new thinner-strut (≤150 µm) BRS technologies.
| Device | Manufacturer | Backbone material & coating | Eluted drug | Strut thickness (µm) | Resorption (months) | CE mark status | Drug dose |
|---|---|---|---|---|---|---|---|
| MeRes100 (current study) | Meril Life Sciences | Material: PLLACoating: PDLLA | Sirolimus | 100 | 24 | CE marked | 1.25 µg/mm2 |
| Absorb BVS gen 2 | Abbott | Material: PLLACoating: PDLLA | Everolimus | <99 | 36 | CE marked | – |
| DESolve Nx | Elixir Medical | Material: PLLACoating: PLLA | Novolimus | 150 | 24 | CE marked 2014 | 5 µg/mm2 |
| DESolve Cx | Elixir Medical | Material: PLLACoating: PLLA | Novolimus | 120 | 24 | CE marked 2017 | 5 µg/mm2 |
| FORTITUDE | Amaranth Medical | Material: PLLACoating: PDLLA | Sirolimus | 150 | 10 | CE marked | 96 mg/cm2 |
| Mirage | Manli Cardiology | Material: PLLACoating: PLLA | Sirolimus | 125 | 14 | CE marked | – |
| Firesorb | MicroPort Medical | Material: PLLACoating: PDLLA | Sirolimus | 100-125 | 36 | CE marked | 4 µg/mm2 |
| Fantom | REVA Medical | Material: PTD-PCCoating: PTD-PC | Sirolimus | 125 | – | CE marked 2017 | 115 µg |
| DREAMS 3G23 | Biotronik | Material: BIOmag-alloyCoating: PLLA | Sirolimus | 99 μm – device diameter 2.5 mm117 μm – device diameters 3.0 mm and 3.5 mm147 μm – device diameter 4.0 mm | 12 | CE marked | 1.4±0.3 μg/mm2 |
| Strut technology details taken from2223. CE: European Conformity; PDLLA: poly-D, L-lactide; PLLA: poly L-lactide; PTD-PC: poly-tyrosine-derived polycarbonate | |||||||
Limitations
The limitations of this study include its retrospective design, the relatively small sample size, lack of imaging follow-up, and only a medium follow-up duration. Reflecting real-life clinical practice, the study sample was highly heterogeneous in terms of lesion characteristics. Therefore, there is an ongoing need for long-term follow-up studies with larger patient populations to fully understand and validate these observations and conclusions.
Conclusions
In conclusion, our preliminary findings indicate that implantation of a thinner-strut sirolimus-eluting BRS presents a promising, safe and reliable solution for the treatment of de novo and in-stent restenotic coronary artery lesions. Future studies involving larger cohorts and long-term follow-ups are necessary to further confirm the benefits and assess the long-term implications of this therapeutic strategy.
Impact on daily practice
Bioresorbable vascular scaffolds are emerging because of the risks of increased major adverse cardiac events (MACE), stent thrombosis, and late restenosis that are associated with drug-eluting stents. The 1-year outcomes of the thin-strut sirolimus-eluting MeRes100 bioresorbable scaffold (BRS) show it to be associated with lower rates of mortality and an absence of MACE. The MeRes100 BRS is useful in treating in-stent restenosis and de novo coronary lesions.
Acknowledgements
The investigators would like to thank the study personnel, Dr Pallvi Verma, Mr Amarnath Yadav and Mr Ankit Thakur, for their contributions to the research and preparation of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.

