Haemopericardium is a major complication of balloon mitral valvotomy (BMV). It results from chamber perforation during transseptal puncture (TSP) or manipulation of the balloon and wire while performing BMV1. In patients with an enlarged left atrium (LA), if the overlapping walls of the right atrium (RA) and LA near the right lateral and inferior borders are punctured, the catheter or needle may perforate the right atrial wall and re-enter the LA through the pericardial space (Figure 1A). This is known as the “stitch phenomenon”. This dangerous situation requires immediate surgical intervention, as blood can enter the pericardial space from both the LA and RA2. A 26-year-old pregnant female at 25 weeks of gestation was experiencing worsening dyspnoea despite treatment with beta blockers and diuretics. On transthoracic echocardiography (TTE), severe mitral stenosis was noted (mitral valve area [MVA] planimetry=0.74 cm2), The peak/mean (P/M) transmitral gradients (TMG) were 51/33 mmHg, respectively. Both commissures were fused. Trivial mitral regurgitation, moderate tricuspid regurgitation, and severe pulmonary arterial hypertension (pulmonary artery systolic pressure=93 mmHg) were also observed. During BMV, transseptal puncture was performed under fluoroscopic guidance, using standard landmarks. The position of the TSP needle within the LA was confirmed with bright red blood, LA pressure waveform, and contrast injection under fluoroscopy (Figure 1B, Moving image 1). A looped LA wire was positioned in the LA. The septal puncture was dilated with a transseptal dilator over the looped LA wire. The patient developed bradycardia with hypotension within seconds of the removal of the septal dilator. The patient became drowsy and tachypnoeic. TTE was suggestive of pericardial effusion with tamponade. Pericardiocentesis with autotransfusion was initiated. The patient responded well to pericardiocentesis, ionotropic support, and a 200 mL normal saline flush and regained consciousness. A double-stitch injury was suspected because of the confirmed TSP needle position in the LA and the rapid tamponade following dilatation with the transseptal dilator. The stitch phenomenon was confirmed by the pull of the transseptal dilator, leading to a simultaneous increase in pericardial effusion and sealing of the effusion after repositioning the septal dilator. Given the high risk associated with emergency surgery, we planned to close the double-stitch injury with a device that was available in the cath lab at that time. The transseptal dilator was replaced with an 8 Fr Amplatzer device delivery sheath (Abbott) over the looped LA wire in the LA. The closure device was loaded over the device delivery cable and passed through the delivery sheath. The double-stitch injury was closed with a 12/7 mm Amplatzer Muscular VSD Occluder (Abbott) device by positioning one disc in the LA and one disc in the RA (Moving image 2). Once there was no effusion and the sealing of the perforation was confirmed, the VSD device was released (Figure 1C—Figure 1D–Figure 1E–Figure 1F–Figure 1G). The patient was moved to the intensive care unit with low-dose inotropic support. There was no increase in pericardial effusion for 24 hours, and inotropes were tapered off. The pericardial sheath was then removed. After one week, a BMV was reattempted, and a TSP was performed under transoesophageal echocardiographic guidance (Figure 1H, Moving image 3). The BMV was performed uneventfully with a 26 mm Accura balloon (Figure 1I, Moving image 4). The mean LA pressure reduced from 38 mmHg to 24 mmHg and the invasively measured pulmonary artery pressure reduced from 65/28 mmHg (mean 47 mmHg) to 50/22 mmHg (mean 32 mmHg). On the post-BMV TTE, the MVA as measured by planimetry was 1.75 cm2, with a P/M TMG of 15/9 mmHg (Figure 1J– Figure 1K). The patient was discharged on tablet Ecosprin 150 mg once a day and low-dose diuretics and was advised admission for observation and a hospital delivery at term gestation. Following transseptal access, the occurrence of a new pericardial effusion is typically attributed to perforation. The manifestation (effusion/tamponade) is contingent upon the device responsible for the perforation (needle/sheath/dilator), the perforated structure, the haemodynamic status, and the coagulation status3. It is difficult to recognise stitch injury with regular parameters such as oximetry, pressure recording, and contrast injection into the LA. After dilatation of the transseptal puncture, haemopericardium occurs and cardiac tamponade ensues; this is precisely what happened in our case. Sealing off the defect after repositioning the septal dilator is confirmatory. The preferred option is to seal the perforation by open heart surgery and simultaneously perform a surgical mitral comissurotomy (open/closed)45. There are reports of sealing a stitch injury perforation with cyanoacrylate glue via a pericardial sheath4. The septal dilator’s diameter is 14 Fr (4.66 mm), and the defect it creates is almost 5 mm in diameter. A device with a waist diameter of 7 mm upwards and disc diameter of 10 mm upwards can be used to adequately seal the defect without any leak. We preferred the Amplatzer Muscular VSD device because its wider waist of 7 mm helped us to close the defects by keeping one rim in the LA and the other in the RA while the waist of the device was in the pericardial space. Such a device can be kept in a cath lab where BMV is routinely performed, in order to tackle such emergencies. At the same time, an interventional cardiologist’s expertise is of paramount importance to deploy the device. In our case, there was a risk of inadequate sealing of the defect, continuing haemopericardium, and the device dislodging into the LA/RA/pericardial cavity, which could have been easily corrected by the otherwise inevitable surgery. This article includes a visual abstract to provide a quick review of the case with images (Supplementary Figure 1) . 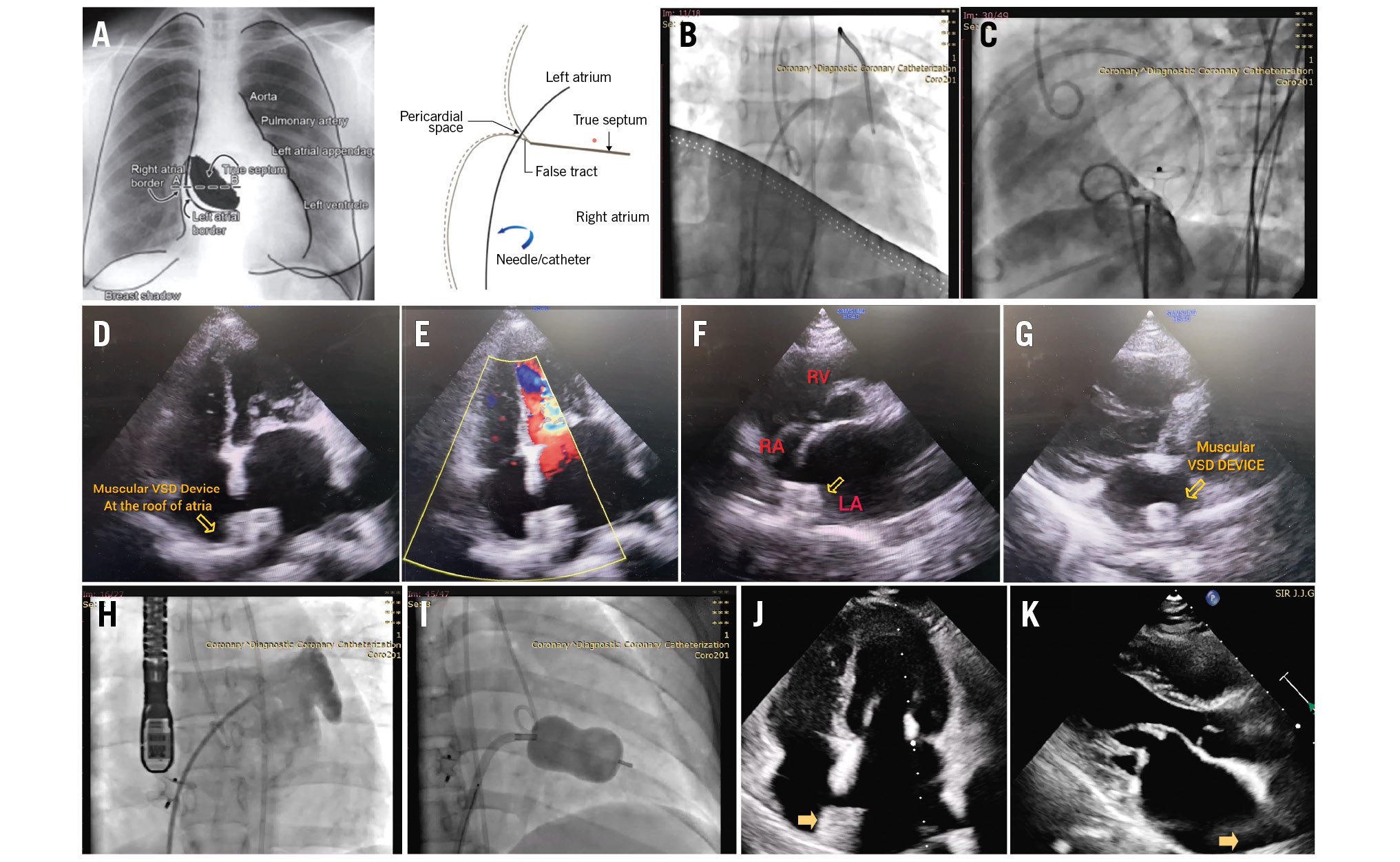
Figure 1. Percutaneous closure of double-stitch injury by Muscular VSD device followed by interval BMV. A) Schematic diagram showing the absence of the true atrial septum near the lateral and inferior borders of the LA and the path traversed by the TSP needle from the RA across the pericardial space to the LA3. B) Dye injection showing the Mullins sheath (Medtronic) positioned in the LA after TSP. C) Dye injection in the RA showed no flow across the Muscular VSD device, which was sealing the double-stitch injury with one disc in the LA and another disc in the RA. D, E) Muscular VSD device placed at the roof of the LA and RA, with no flow across it in the apical four-chamber view. F) Device across the IAS seen in the PSAX view. G) Muscular VSD device seen in the LA in the PLAX view. H) TOE-guided TSP during interval BMV. I) Accura balloon inflated across the MV. J, K) Post-BMV TTE showing a well-opening mitral valve and the device in the LA. BMV: balloon mitral valvotomy; IAS: interatrial septum; LA: left atrium; PLAX: parasternal long-axis; PSAX: parasternal short-axis; RA: right atrium; TSP: transseptal puncture; TTE: transthoracic echocardiography; TOE: transoesophageal echocardiography; VSD: ventricular septal defect
Conflict of interest statement
The authors have no conflicts of interest to declare.

