The prevalence of coronary bifurcation lesions varies between 15% and 40% of all percutaneous coronary interventions (PCI), depending on the definitions applied12. Stenting coronary bifurcation lesions presents a technical challenge compared to non-bifurcations and is associated with worse clinical outcomes, including higher rates of revascularisation and stent thrombosis, particularly when systematic two-stent approaches are used1234. Consequently, the 2018 European Society of Cardiology (ESC) guidelines on myocardial revascularisation recommend provisional stenting (main vessel stenting with provisional side branch stenting, if necessary) for the majority of bifurcation lesions5. Despite this, controversy persists regarding the clinical outcomes of different stenting approaches. Due to the small sample sizes, varied follow-up duration, and inconsistent endpoint definitions in randomised clinical trials, multiple meta-analyses34678910111213 have been conducted to compare provisional stenting with upfront two-stent approaches. However, these meta-analyses − limited by study-level data, significant heterogeneity3467891011, inclusion of different types (false or true bifurcations) and locations (distal left main and non-left main) of bifurcation lesions, and a paucity of long-term (greater than five years) clinical outcomes − have yielded inconclusive results. Moreover, the lack of stratification based on lesion complexity34789101112 has hindered the understanding of how lesion complexity influences the comparative efficacy of stenting techniques. Although study-level meta-analyses have suggested that double-kissing crush (DK crush) stenting is associated with fewer adverse clinical events, its long-term (six-year) benefits remain unclear. This individual patient data (IPD) analysis based on randomised clinical trials with reduced heterogeneity aims to assess the long-term advantages of systematic two-stent techniques (particularly DK crush) over provisional stenting for true coronary bifurcation lesions.
Methods
This systematic review and IPD-level analysis of randomised trials with centrally adjudicated endpoints were conducted to evaluate the comparative effectiveness and safety of systematic two-stent techniques versus provisional stenting in patients with true coronary bifurcation lesions treated with a drug-eluting stent. All patients were followed prospectively and had interventions occurring at least six years earlier. Trials comparing different two-stent techniques or involving intravascular imagining guidance versus angiography guidance were excluded. No additional restrictions were applied for trial selection. This IPD analysis follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (www.prisma-statement.org), and the study protocol was prospectively registered with PROSPERO (DKCRUSH X Study, www.crd.york.ac.uk/prospero: CRD42024580040).
Search strategy
Two investigators (J. Kan, K.S. Lee) independently determined trial eligibility, with a third investigator (S.-L. Chen) resolving any disagreement. We searched Ovid Medline, PubMed, and Embase on 28 August 2024, to identify randomised controlled trials. Reference lists of identified articles were manually reviewed to locate additional studies. No language restrictions were applied. Studies were included based on the following criteria: (1) randomised controlled clinical trial comparing systematic two-stent versus provisional stenting; (2) true bifurcation lesions (Medina 1,1,1/1,0,1/0,1,1); (3) side branch lesion length ≥10 mm; (4) ≥6 years follow-up; and (5) use of drug-eluting stents. Eligible trials were predominantly identified from the DKCRUSH/DEFINITION consortium to ensure technical consistency in two-stent approaches. Studies outside this consortium were excluded due to heterogeneity in procedural techniques or endpoint definitions.
Study outcomes
The primary endpoint was target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (MI; both periprocedural and spontaneous), or clinically driven target lesion revascularisation at six years. Only centrally adjudicated events by an independent clinical events committee were included in the analysis. Secondary endpoints included TLF without periprocedural MI, all-cause death, cardiac death, periprocedural and spontaneous MI, target lesion and target vessel revascularisation, and stent thrombosis (definite and probable). For patients lost to follow-up, the last known follow-up was used as the final result.
Data extraction and quality assessment
The principal investigators of eligible trials provided anonymised IPD in an electronic dataset for analysis. In case of missing data or inconsistencies, the principal investigators of the trials were contacted and asked to provide source documents, which were reviewed by a newly established clinical event committee. Two investigators (J. Kan, K.S. Lee) independently assessed the risk of bias using the revised Cochrane risk-of-bias tool (RoB 2). Disagreements were resolved through discussion or arbitration by a third author (S.-L. Chen). Only full-text, peer-reviewed studies (with a mean lesion length in the side branch at least of 10 mm) were included, and reference lists of retrieved articles were manually screened for additional studies not captured in the initial search. Each trial was approved by the local medical ethics committee, and all patients provided written informed consent.
Statistical analysis
A one-step approach was used to model patient-level data from all included trials simultaneously, employing a mixed-effect Cox regression model with baseline hazards stratified by trial and a random slope to account for variation between trials in treatment effects. Treatment effects were expressed as a hazard ratio (HR) and 95% confidence interval (CI). The heterogeneity of the treatment effect between trials was quantified using the variance of the random slope (τ²). Sensitivity analyses employed a two-step approach using the Paule-Mandel estimator to combine trial-level estimates, with heterogeneity measured by I2. We first assessed the difference in the primary endpoint between the systematic two-stent approach and provisional stenting in the intention-to-treat population, adjusted by sex, initial presentations at admission (chronic coronary syndrome, unstable angina, and myocardial infarction), diabetes, and participating sites (China, other Asian countries, and Europe/America). The intention-to-treat population included all randomised patients according to their random allocation. The per-protocol population excluded patients who were ineligible due to protocol violations or who did not receive the assigned treatment strategy. Lesions were also stratified by lesion complexity using the DEFINITION criteria14. Primary and secondary endpoints were compared between provisional stenting and upfront two-stent groups for patients with simple or complex bifurcations. Prespecified subgroup analyses included age (>65 vs ≤65 years), sex, diabetes status, renal dysfunction (defined as estimated glomerular filtration rate <60 vs ≥60 mL/min/1.73 m²), heart failure, initial presentations at admission (chronic coronary syndrome vs unstable angina vs MI), hypertension, hyperlipidaemia, single- versus multivessel disease, complete versus incomplete revascularisation, and use of intravascular imaging guidance. The analyses were performed using R, version 4.3.1 (R Foundation for Statistical Computing). The Review Manager (RevMan) version 5.4 (The Cochrane Collaboration) was used to calculate the pooled effect sizes. Significant heterogeneity was defined as I2 >50% or a p-value for heterogeneity <0.05. Publication bias was assessed using funnel plots. For all analyses, a two-sided p-value and 95% confidence interval were reported.
Results
A total of 6,225 citations were screened. Of those, 610 records were considered potentially eligible during the screening of titles and abstracts, and four trials were deemed eligible and provided IPD (Figure 1): the DKCRUSH-II15, DKCRUSH-IV16, DKCRUSH-V17, and DEFINITION II18 trials. The characteristics, main inclusion or exclusion criteria, and definition of primary endpoints of the trials were the same across the four trials. The risk of bias assessment revealed no major concerns. All four trials were sponsored by non-profit academic organisations. The final patient enrolment in the DEFINITION II trial occurred on 8 November 2018, establishing the scheduled clinical follow-up for this IPD analysis to be completed on 8 November 2024. In total, 1,573 patients were included in the intention-to-treat analysis (Central illustration), with 791 (50.3%) patients assigned to the systematic two-stent approach and 782 (49.7%) to the provisional stenting group. Baseline clinical characteristics were balanced between the groups (Table 1). The median age was 65.0 years, and 349 (22.2%) participants were female. A total of 457 (29.1%) patients had a history of diabetes, and 246 (15.6%) had renal dysfunction. Additionally, 228 (14.5%) had a history of heart failure, including 81 (5.1%) with a left ventricular ejection fraction of less than 40%. A history of MI, PCI, or coronary artery bypass graft surgery was present in 248 (15.8%), 282 (17.9%), and 7 (0.4%) patients, respectively. At presentation, 345 (10.9%) patients had chronic coronary syndrome, 943 (59.9%) had unstable angina, 122 (7.8%) had ST-segment elevation MI (STEMI), and 162 (10.3%) had non-STEMI. A total of 736 (46.8%) patients had distal left main bifurcation lesions, and 1,085 (69.0%) had multivessel disease. Thrombus-containing lesions were identified in 112 (7.2%) patients, and Medina 1,1,1 bifurcation lesions were present in 1,199 (76.2%) patients. A total of 956 (60.8%) patients had a SYNTAX score >22. A total of 866 (55.1%) lesions were classified as complex according to the DEFINITION criteria14. The transradial approach was used in 1,118 (71.1%) patients, and an intravascular imaging-guided procedure was performed in 705 (44.8%) patients, with intravascular ultrasound being the most common guidance (37.8%). Predilation for the side branch was performed in 314 (40.2%) patients in the provisional stenting group, compared to 53.8% in the two-stent group. The median stent length was 38 mm in the main vessel and 20 mm in the side branch, which corresponded to the mean lesion lengths (31.9 mm and 17.1 mm, respectively). Two-stent techniques were ultimately used in the provisional stenting group in 258 (33.0%) patients. In the two-stent group, provisional stenting was used in 35 (4.4%) patients, while the remaining 756 (95.6%) received two stents, with DK crush used in 652 (86.2%). Final kissing balloon inflation was performed in 94.6% (two-stent group) versus 65.7% (provisional stenting group). Use of the proximal optimisation technique was significantly higher in the two-stent group (43.2%) than in the provisional stenting group (26.9%; p<0.001). Thrombolysis in Myocardial Infarction grade 3 flow in both the main vessel and side branch was achieved in 1,533 (97.5%) patients post-procedure. The median contrast volume and procedural time were significantly greater in the two-stent group (200 [interquartile range [IQR] 150, 260] mL and 66 [IQR 42, 90] min) than in the provisional stenting group (180 [IQR 120, 240] mL; p<0.001; and 56 [IQR 34, 81] min; p<0.001). Dual antiplatelet therapy (DAPT) was prescribed to 1,542 (98.0%) patients at discharge, 1,394 (88.6%) at one year, 713 (45.3%) at three years, and 480 (30.5%) at six years. At a median of 365 days, 920 (58.5%) patients underwent angiographic follow-up, and restenosis in the side branch was found to have occurred in 107 (24.0%) patients in the provisional group and 70 (15.2%) in the two-stent group (p=0.0005). At six years, TLF had occurred in 144 (18.2%) patients in the two-stent group, compared with 193 (24.7%) in the provisional stenting group (HR 0.71, 95% CI: 0.57-0.89; τ²=0.00; I²=0%; p=0.0022) (Table 2, Figure 2). Subgroup analyses showed no significant interactions (Figure 3). Target lesion revascularisation and target vessel myocardial infarction rates were lower in the two-stent group (88 [11.1%] and 30 [3.8%], respectively) compared to the provisional stenting group (118 [15.1%], HR 0.73, 95% CI: 0.55-0.96; p=0.0258, and 67 [8.6%], HR 0.44, 95% CI: 0.29-0.68; p=0.0002, respectively) (Table 2). TLF excluding periprocedural myocardial infarction was 17.3% in the two-stent group and 21.9% in the provisional stenting group (HR 0.78, 95% CI: 0.62-0.97; p=0.0275). These results were consistent with unadjusted analyses. A total of 63 (4.0%) participants (35 in the two-stent group and 28 in the provisional stenting group) were excluded from the per-protocol analysis due to non-compliance with the allocated treatment strategy. In the per-protocol analysis, the composite of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation were lower in the systematic two-stent group (n=136, 18.0%) than in the provisional stenting group (n=189, 25.1%; p=0.0012). Cardiac death and stent thrombosis rates (Table 2) were similar between the two groups across all analyses. According to the DEFINITION criteria, patients with complex bifurcation lesions exhibited higher rates of hyperlipidaemia, diabetes, smoking, stroke, and MI at initial presentation. The rate of target lesion failure was similar between the provisional stenting and two-stent groups in patients with simple bifurcations. However, the two-stent approach resulted in a lower incidence of target vessel myocardial infarction (2.3%) compared to 6.0% in the provisional stenting group (p=0.0241) among patients with simple bifurcations, without significant differences in target lesion revascularisation. Among patients with complex coronary bifurcation lesions, TLF occurred in 88 (19.6%) patients in the two-stent group versus 115 (27.6%) in the provisional stenting group (HR 0.68, 95% CI: 0.52-0.90; p=0.0066). This reduction was primarily driven by lower rates of target lesion revascularisation (10.5% [n=47] in the two-stent group vs 16.1% [n=67] in the provisional stenting group, HR 0.64, 95% CI: 0.44-0.93; p=0.0199) and target vessel myocardial infarction (4.9% [n=22] vs 10.8% [n=45], HR 0.46, 95% CI: 0.27-0.76; p=0.0027). Among patients with simple coronary bifurcation lesions, periprocedural MI occurred in 2 (0.6%) patients in the two-stent group versus 5 (1.4%) in the provisional stenting group (HR 0.43, 95% CI: 0.08-2.20; p=0.308), while acute side branch occlusion was comparable between the two-stent and provisional stenting groups (1.2% vs 1.6%; p=0.892). 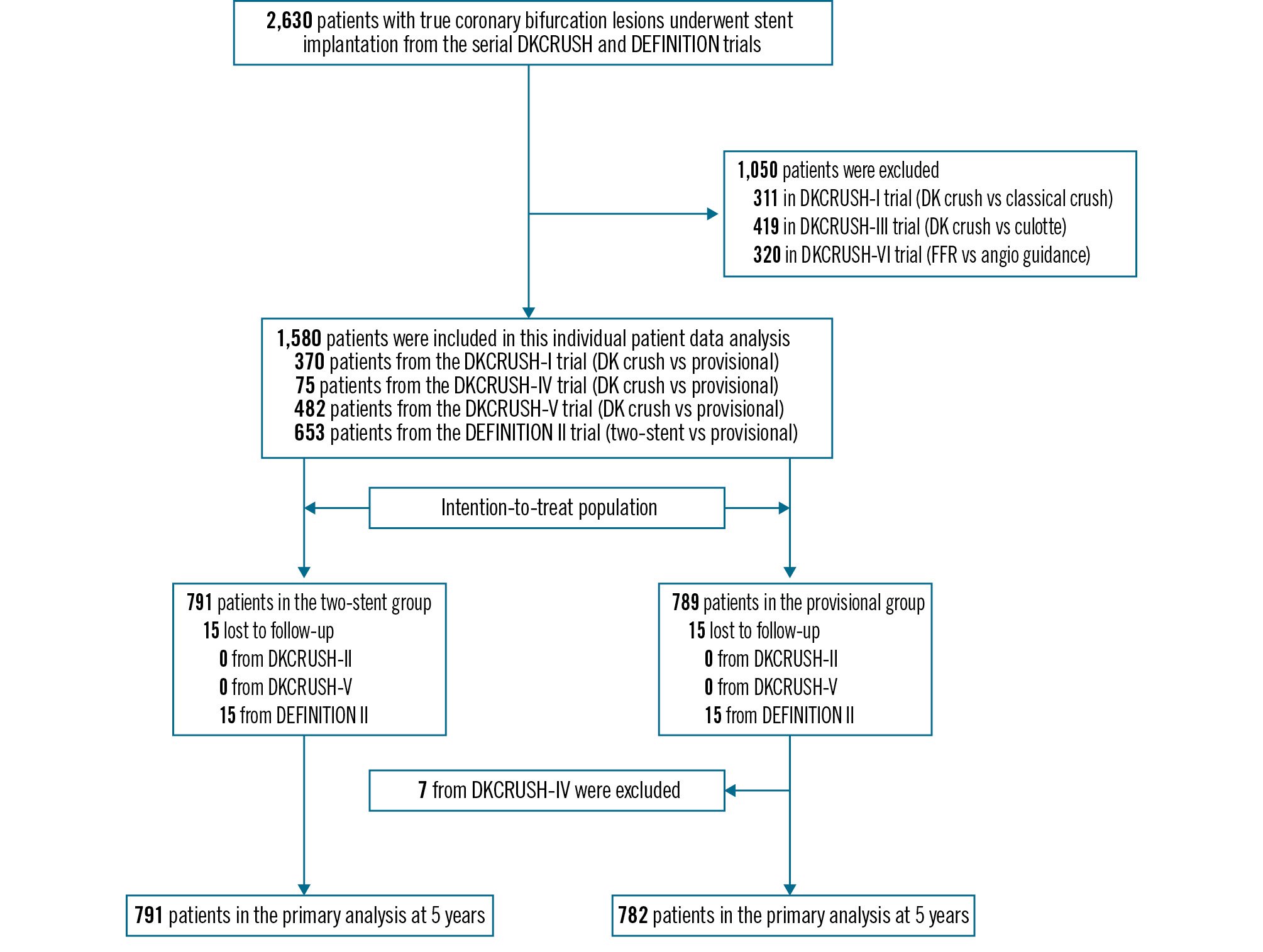
Figure 1. DKCRUSH X study flowchart. Of a total of 1,580 screened patients, 1,573 were included in the intention-to-treat analysis. DKCRUSH-II: a randomised clinical study comparing DK crush with provisional stenting for treatment of coronary bifurcation lesions: results from the DKCRUSH-II (Double Kissing Crush versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions) trial; DKCRUSH-IV: haemodynamic changes of fractional flow reserve after double kissing crush and provisional stenting technique for true bifurcation lesions; DKCRUSH-V: Double Kissing Crush Versus Provisional Stenting for Left Main Distal Bifurcation Lesions: DKCRUSH-V Randomised Trial; DEFINITION II: multicentre, randomised comparison of two-stent and provisional stenting techniques in patients with complex coronary bifurcation lesions: the DEFINITION II trial. DK crush: double-kissing crush; FFR: fractional flow reserve.
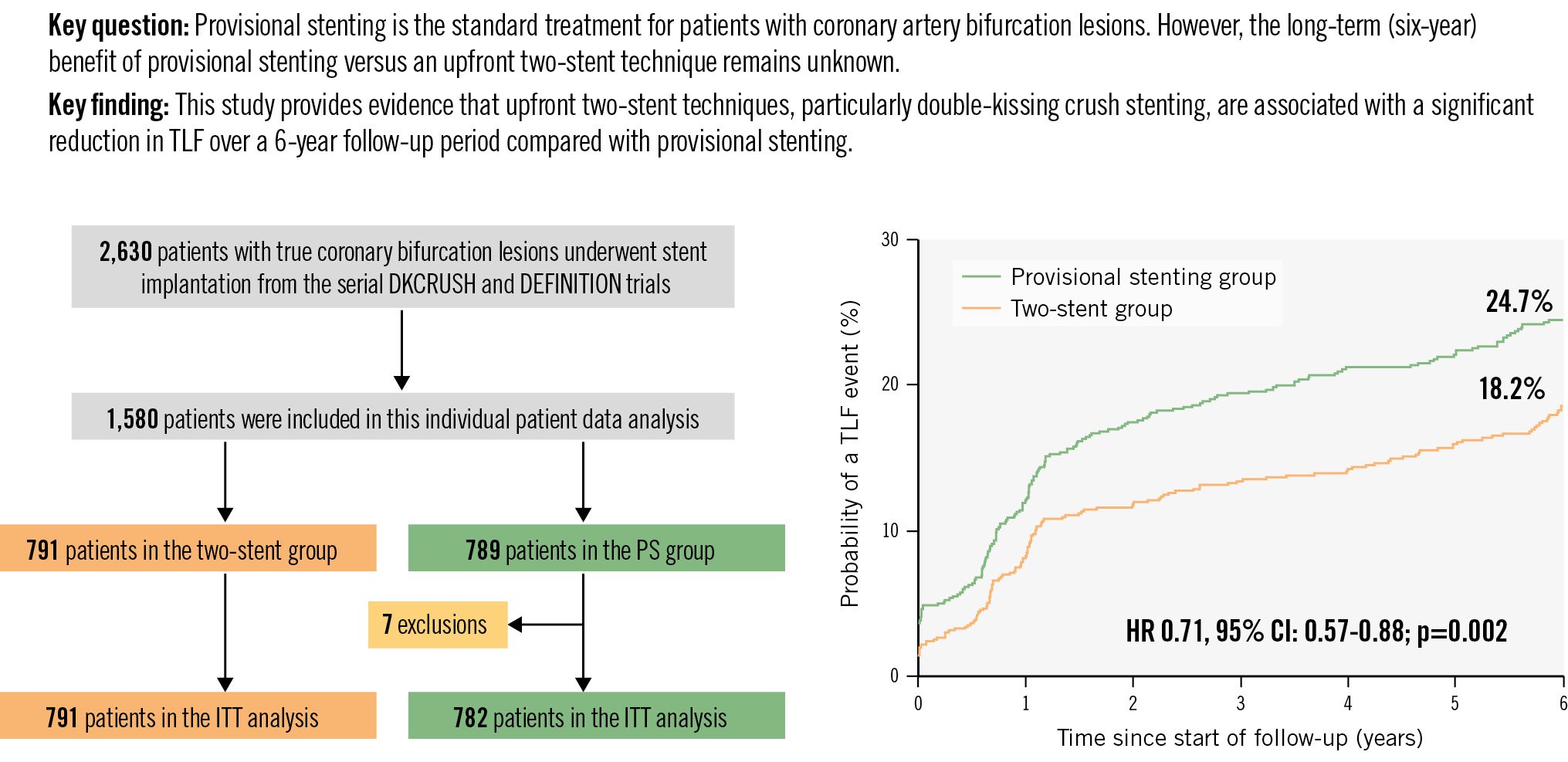
Central illustration. Long-term outcomes of DK crush vs provisional stenting for bifurcations. Of 2,630 patients with true coronary artery bifurcation lesions, 1,573 patients were studied. At six-year follow-up, target lesion failure occurred in 24.7% of patients in the provisional group and 18.2% in the upfront two-stent group. CI: confidence interval; HR: hazard ratio; ITT: intention-to-treat; PS: provisional stenting; TLF: target lesion failure
Table 1. Baseline clinical characteristics.
| Overall population (n=1,573) | Two-stent group (n=791) | Provisional stenting group (n=782) | p-value | |
|---|---|---|---|---|
| Included studies | ||||
| DKCRUSH-II1526 | 370 (23.5) | 185 (23.4) | 185 (23.7) | |
| DKCRUSH-IV16 | 68 (4.3) | 38 (4.8) | 30 (3.8) | |
| DKCRUSH-V17 | 482 (30.6) | 240 (30.3) | 242 (30.9) | |
| DEFINITION II18 | 653 (41.5) | 328 (41.5) | 325 (41.6) | |
| Geographical region | ||||
| China | 924 (58.7) | 469 (59.3) | 455 (58.2) | 0.655 |
| Other Asian countries | 284 (18.0) | 134 (16.9) | 150 (19.2) | 0.248 |
| Europe and America | 365 (23.3) | 188 (23.8) | 177 (22.6) | 0.595 |
| Age, years | 65 (57, 72) | 65 (57, 72) | 65 (57, 72) | 0.985 |
| Female sex | 349 (22.2) | 168 (21.2) | 181 (23.1) | 0.363 |
| Height, cm | 168 (162, 172) | 168 (162, 172) | 168 (162, 172) | 0.394 |
| Weight, kg | 70 (62, 75) | 70 (62, 75) | 70 (62, 75) | 0.497 |
| Body mass index, kg/m2 | 24.57 (22.84, 26.67) | 24.57 (22.86, 26.67) | 24.57 (22.84, 26.67) | 0.647 |
| Hypertension | 1,070 (68.0) | 560 (70.8) | 510 (65.2) | 0.018 |
| Hyperlipidaemia | 783 (49.8) | 380 (48.0) | 403 (51.5) | 0.166 |
| Renal dysfunction* | 246 (15.6) | 123 (15.5) | 123 (15.7) | 0.922 |
| Diabetes | 457 (29.1) | 225 (28.4) | 232 (29.7) | 0.593 |
| On insulin treatment | 128 (28.0) | 63 (28.0) | 65 (28.0) | 0.997 |
| Current smoker† | 431 (27.4) | 232 (29.3) | 199 (25.4) | 0.084 |
| Previous PCI | 282 (17.9) | 145 (18.3) | 137 (17.5) | 0.675 |
| Previous CABG | 7 (0.4) | 2 (0.3) | 5 (0.6) | 0.285 |
| Previous MI | 248 (15.8) | 128 (16.2) | 120 (15.3) | 0.649 |
| GI bleeding | 30 (1.9) | 18 (2.3) | 12 (1.5) | 0.283 |
| Stroke | 108 (6.9) | 44 (5.6) | 64 (8.2) | 0.04 |
| Peripheral artery disease | 115 (7.3) | 57 (7.2) | 58 (7.4) | 0.872 |
| Heart failure | 228 (14.5) | 110 (13.9) | 118 (15.1) | 0.505 |
| LVEF, % | 62.0 (55.0, 66.0) (n=1,244) | 62.0 (55.0, 65.5) (n=619) | 62.0 (56.0, 66.0) (n=625) | 0.208 |
| LVEF <40% | 81 (5.1) | 42 (5.3) | 39 (5.0) | 0.772 |
| Red blood cells, 109/L | 4.43 (4.06, 4.78) | 4.41 (4.04, 4.75) | 4.45 (4.08, 4.82) | 0.147 |
| White blood cells, 109/L | 6.66 (5.50, 8.00) | 6.60 (5.53, 8.00) | 6.70 (5.47, 8.07) | 0.826 |
| Total cholesterol, mg/dL | 159 (131, 189) | 159 (128, 189) | 159 (131, 189) | 0.52 |
| Low-density lipid cholesterol, mg/dL | 93 (72, 120) | 92 (70, 120) | 94 (73, 120) | 0.24 |
| Fasting blood glucose, mg/dL | 97 (88, 121) | 95 (87, 115) | 99 (88, 124) | 0.126 |
| Serum creatinine, mg/dL | 0.90 (0.76, 1.07) | 0.91 (0.77, 1.07) | 0.90 (0.76, 1.07) | 0.347 |
| Presentation on admission | ||||
| Silent ischaemia | 73 (4.6) | 33 (4.2) | 40 (5.1) | 0.374 |
| Stable angina | 272 (17.3) | 145 (18.3) | 127 (16.2) | 0.273 |
| Unstable angina | 943 (59.9) | 466 (58.9) | 477 (61.0) | 0.399 |
| STEMI | 122 (7.8) | 67 (8.5) | 55 (7.0) | 0.287 |
| NSTEMI | 162 (10.3) | 79 (10.0) | 83 (10.6) | 0.683 |
| Medications on admission | ||||
| Statin | 1,146 (72.9) | 571 (72.2) | 575 (73.5) | 0.643 |
| ß-blocker | 293 (18.6) | 137 (17.3) | 156 (19.9) | 0.401 |
| ACEi/ARB | 337 (21.4) | 177 (22.4) | 160 (20.5) | 0.058 |
| Calcium channel blocker | 233 (14.8) | 106 (13.4) | 127 (16.2) | 0.242 |
| Data are n (%) or median (interquartile range). *Defined as eGFR <60 mL/min/1.73 m2. †Defined as those who had smoked ≥100 lifetime cigarettes and were still smoking at the time of enrolment; other tobacco products were not included. ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CABG: coronary artery bypass graft; eGFR: estimated glomerular filtration rate; GI: gastrointestinal; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction | ||||
Table 2. Clinical outcomes in the intention-to-treat population.
| Two-stent group (n=791) | Provisional stenting group (n=782) | Hazard ratio* (95% CI) | p-value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Target lesion failure, a composite of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation | 144 (18.2) | 193 (24.7) | 0.71 (0.57 to 0.89) | 0.0022 |
| Secondary outcomes | ||||
| Target lesion failure without periprocedural myocardial infarction | 137 (17.3) | 171 (21.9) | 0.78 (0.62 to 0.97) | 0.0275 |
| Cardiac death | 53 (6.7) | 55 (7.0) | 0.96 (0.66 to 1.39) | 0.815 |
| All-cause death | 101 (12.8) | 107 (13.7) | 0.93 (0.71 to 1.22) | 0.605 |
| Target vessel myocardial infarction | 30 (3.8) | 67 (8.6) | 0.44 (0.29 to 0.68) | 0.0002 |
| Periprocedural myocardial infarction | 10 (1.3) | 27 (3.5) | –2.2% (–3.8% to –0.7%) |
0.0042 |
| Spontaneous myocardial infarction | 20 (2.5) | 40 (5.1) | 0.49 (0.28 to 0.83) | 0.0086 |
| Clinically driven target lesion revascularisation | 88 (11.1) | 118 (15.1) | 0.73 (0.55 to 0.96) | 0.0258 |
| Clinically driven target vessel revascularisation | 93 (11.8) | 123 (15.7) | 0.74 (0.57 to 0.97) | 0.0286 |
| Stent thrombosis | 36 (4.6) | 45 (5.8) | 0.79 (0.51 to 1.22) | 0.282 |
| Definite stent thrombosis | 16 (2.0) | 14 (1.8) | 1.13 (0.55 to 2.31) | 0.742 |
| Probable stent thrombosis | 20 (2.5) | 31 (4.0) | 0.63 (0.36 to 1.11) | 0.111 |
| Data are n (%). *Adjusted by sex, initial presentations at admission (chronic coronary syndrome, unstable angina, and myocardial infarction), diabetes, and participating sites (China, other Asian countries, and Europe/America). CI: confidence interval | ||||
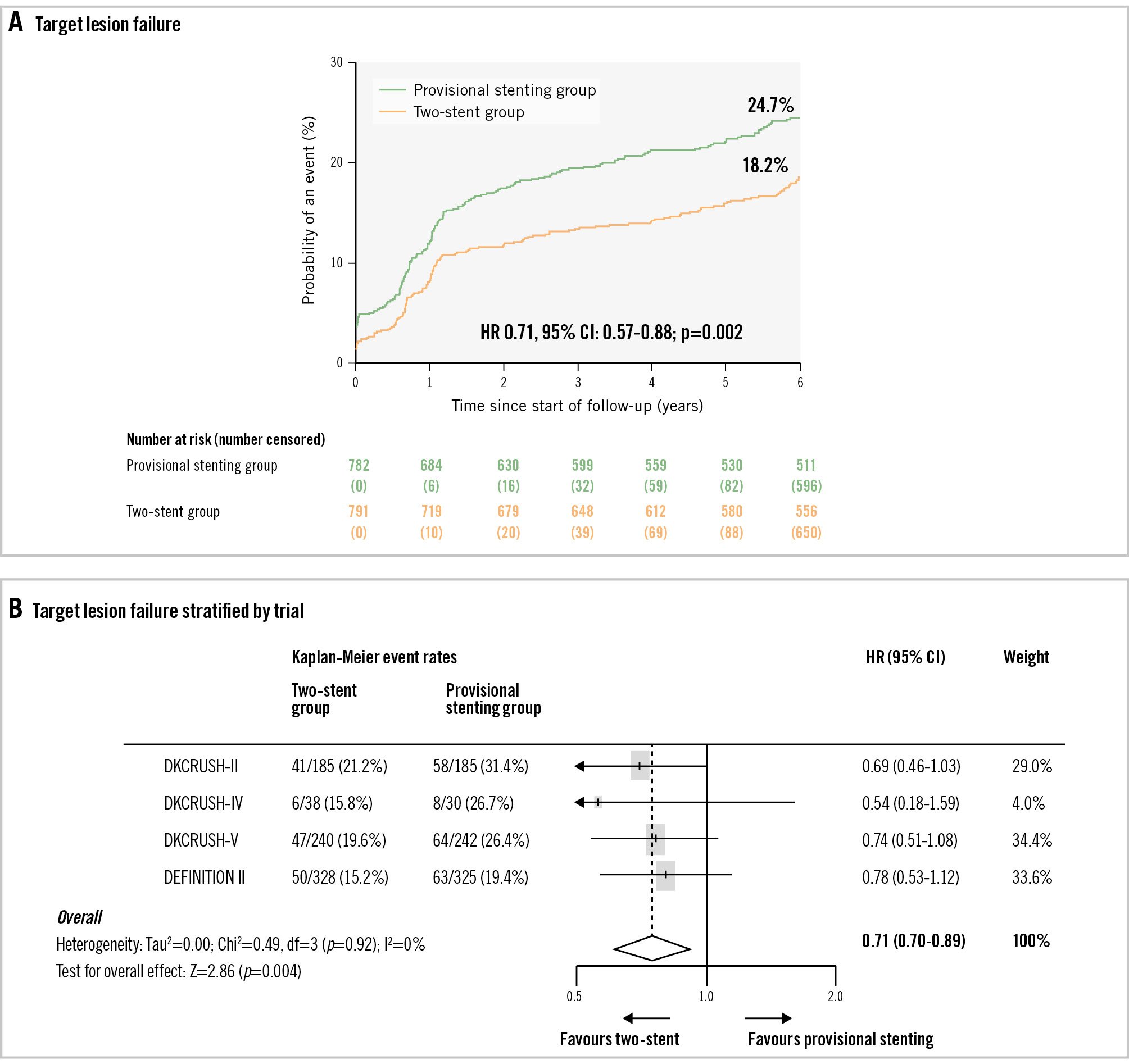
Figure 2. Target lesion failure. A) The rate of target lesion failure at six years after a stenting procedure was 18.2% in the upfront two-stent group and 24.7% in the provisional stenting group (p=0.0022). B) Target lesion failure stratified by trial; the heterogeneity of trials is shown (Tau2=0.00; chi2=0.49, df=3 [p=0.92]; I2=0%; test for overall effect: Z=2.86 [p=0.004]). CI: confidence interval; HR: hazard ratio.
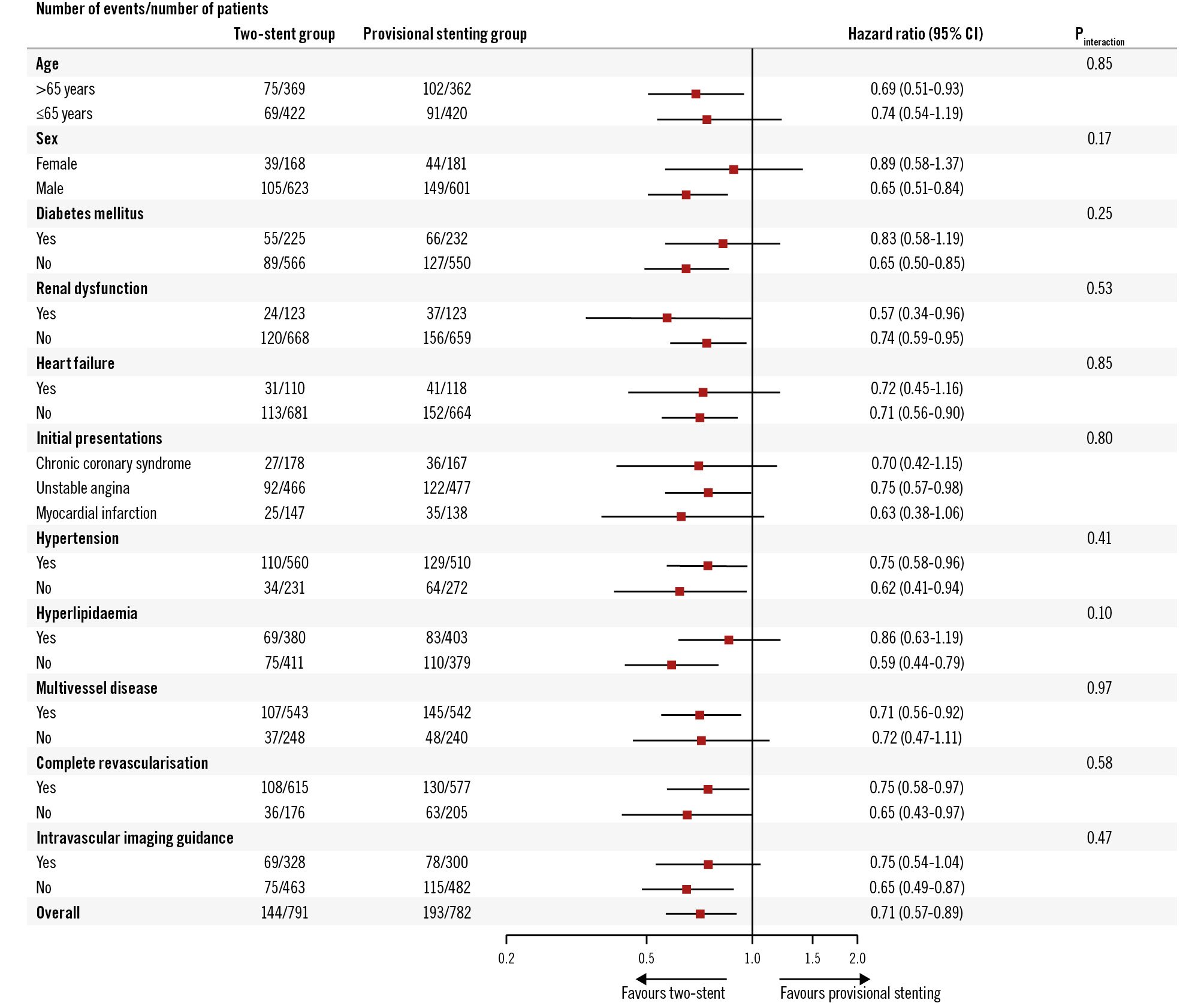
Figure 3. Subgroup analysis on the intention-to-treat population. In the 10 subgroup analyses, no interaction was detected. CI: confidence interval
Discussion
This IPD analysis comprehensively assessed the evidence from randomised clinical trials comparing provisional stenting and systematic two-stent techniques, particularly the DK crush approach, in patients with true coronary artery bifurcation lesions treated with drug-eluting stents. Our findings indicate a significant superiority of the systematic two-stent approach over provisional stenting in reducing the composite endpoint of TLF over a six-year follow-up period. Specifically, two-stent techniques demonstrated considerable reductions in both target vessel myocardial infarction and target lesion revascularisation. Notably, a 28.9% reduction in the risk of six-year TLF was observed in patients with complex bifurcation lesions undergoing two-stent intervention, while no significant benefit was noted for those with simple lesions. Coronary artery bifurcation lesions present a unique challenging lesion subset1234, influenced by various anatomical factors that impact stenting strategies and clinical outcomes514. Existing randomised clinical trials have compared the efficacy of provisional stenting versus systematic two-stent techniques15171819202122, or they have compared different two-stent techniques232425. Yet, discrepancies in study design, lesion complexity, disease burden, amount of myocardium at risk, patient population, operator experience, and technical approaches have hindered the extrapolation of results across trials. To this end, numerous meta-analyses34678910111213 have attempted to address this heterogeneity; however, their reliance on study-level data and varied methodologies has yielded inconclusive results. For instance, the degree of heterogeneity (I2) in prior clinical trials ranged widely (from 0% to 86%)34678910111213; patients with distal left main bifurcation lesions were the only participants in the DKCRUSH-III23, DKCRUSH-V17, and EBC MAIN trials22, but they were excluded in other trials; periprocedural MI was not reported in some trials. Such differences indicate significant variability in treatment effects. The importance of conducting an IPD analysis is underscored by these limitations, particularly as we established an independent clinical event committee to ensure precise adjudication of clinical events, especially regarding periprocedural MI. Prior studies have not consistently demonstrated the superiority of two-stent techniques over the provisional approach, and the latter is often favoured because of concerns regarding prolonged procedure time, greater metal burden, increased contrast use, and heightened radiation exposure202125. Currently, the provisional approach, encapsulated by the philosophy of “keep it simple and safe”, remains the standard approach for most simple bifurcated lesions15. However, our analysis revealed a sustained reduction in TLF over six years for patients in the upfront two-stent group, predominantly due to reductions in target vessel myocardial infarction and target lesion revascularisation. There were no significant differences in cardiac or all-cause mortality between the two groups. Provisional stenting is a stepwise strategy where 33% of cases required bailout two-stenting, reflecting real-world adaptability but also indicating that lesion complexity may dictate strategy selection. This dynamic aspect was inherent in the included trials. The debate regarding different treatment effects among various two-stent approaches has persisted since the era of bare metal stents134111213. Most previous study-level meta-analyses indicating a reduction in clinical events associated with two-stent techniques111213 were primarily driven by trials incorporating the DK crush technique. This technique has been linked to lower rates of restenosis in the side branch due to the introduction of a first kissing balloon inflation, which mitigates complications such as carina and/or plaque shifting1517 and side branch recoiling1415171823, which are commonly associated with provisional stenting. This rationale informed our criteria for including trials where a minimum of 50% of patients underwent DK crush stenting. Importantly, the risk reduction in the primary endpoint associated with the two-stent approach was consistent throughout the six-year follow-up, aligning with the five-year results of the DKCRUSH-II trial26 and the three-year outcomes of the DEFINITION II27 trial. In cases of significant side branch disease (e.g., >50% diameter stenosis or fractional flow reserve ≤0.80) extending over 5 mm, an upfront two-stent strategy is warranted128. However, the lack of universally accepted criteria for defining the complexity of coronary bifurcation lesions complicates clinical decision-making. The DEFINITION criteria14 provide a novel framework for differentiating simple from complex bifurcations, with key components such as a side branch lesion length ≥10 mm. Study-level meta-analyses61329 have corroborated these findings, indicating that longer side branch lesions correlate with greater benefits from two-stent strategies. Our IPD analysis further substantiates that the reduction in TLF associated with two-stent approaches is significant only in complex bifurcations, primarily driven by substantial decreases in target vessel myocardial infarction and target lesion revascularisation. Interestingly, we also observed a 61.7% reduction in target vessel myocardial infarction risk for patients with simple bifurcation lesions undergoing two-stent approaches, a finding not previously reported, though its clinical relevance warrants further investigation in larger cohorts.
Limitations
This IPD analysis has limitations. First, the majority of investigators involved in the four DKCRUSH trials were experienced in both provisional and DK crush stenting techniques1718, potentially limiting the generalisability of our findings to operators with less experience in DK crush stenting. Second, the mean lesion length ≥10 mm in the side branch was a key inclusion criterion to ensure that more complex bifurcation lesions were represented in this IPD analysis consisting of four trials in which DK crush was used in over 50% of patients. Consequently, the higher event rates associated with the other two-stent techniques may have been diluted by the inclusion of DK crush stenting. Future clinical trials comparing DK crush with other two-stent strategies for complex bifurcation lesions are therefore warranted. Third, routine repeat angiography was not performed at six years; however, the clinically driven nature of revascularisation mitigates potential biases associated with visual assessments. Fourth, intravascular imaging guidance was utilised in less than 50% of patients, yet this was consistent across both groups, allowing us to exclude its influence on treatment outcomes. While optical coherence tomography-guided PCI was associated with fewer major adverse cardiac events for coronary bifurcation lesions – most of which were simple, as indicated by a side branch lesion length of 8.9±6.1 mm30 – future randomised trials comparing angiography-guided versus intravascular imaging-guided bifurcation stenting are warranted31. Fifth, the low rate of DAPT at six years in both groups may have impacted stent thrombosis rates, although current guidelines do not advocate for prolonged DAPT after bifurcation stenting32. Sixth, we did not differentiate between DK crush and other two-stent techniques within the upfront two-stent group, as DK crush was employed in the majority of cases. Previous trials have indicated that DK crush yields lower clinical event rates compared to classical crush33 or culotte stenting23 for distal left main bifurcation lesions. Seventh, while we did not compare the outcomes of one-stent versus two-stent strategies in the provisional group, the transition (36.2%) from one to two stents often reflects lesion complexity, necessitating a larger sample size for direct comparisons. Eighth, trials like EBC MAIN and EBC TWO (ClinicalTrials.gov: NCT01560455) were excluded as they did not meet the inclusion criteria (e.g., lack of long-term follow-up or mixed lesion types). This may limit generalisability to all true bifurcations. Ninth, bleeding events were not systematically adjudicated across trials, precluding safety analysis of prolonged DAPT. Finally, the clinical significance of periprocedural MI remains underexplored. However, the lower rate of TLF observed in the two-stent group, even in the absence of periprocedural MI, compared to the provisional stenting group, underscores the net benefits of the upfront two-stent approach, particularly DK crush stenting. This finding suggests that the advantages of the two-stent strategy extend beyond procedural success and highlight its potential for long-term clinical benefits.
Conclusions
In complex true bifurcations defined by the DEFINITION criteria, upfront DK crush significantly reduces TLF compared to provisional stenting.
Impact on daily practice
This individual patient data analysis highlights a significant reduction in target lesion failure associated with the upfront two-stent strategy compared to provisional stenting. The clinical advantages of double-kissing crush stenting are particularly pronounced in true bifurcation lesions with a long lesion in the side branch (>10 mm).
Guest Editor
This paper was guest edited by Davide Capodanno, MD, PhD; A.O.U. Policlinico “G. Rodolico-San Marco”, University of Catania, Catania, Italy.
Acknowledgements
We appreciate all staff participating in data collection and remote monitoring. We thank Professor Feng Chen for leading the statistical team that performed the statistical analysis. S.-L. Chen made substantial contributions to the initial conception and design of the whole study, data management, statistical expertise, and wrote the first draft. J. Kan, J. Zhang, T. Santoso, T.W. Kwan, I. Sheiban, T.S. Rab, M. Munawar, W.-H. Yin, Y. Ye, L. Chen, and K.S. Lee provided comments and suggestions in critical revision of the article. All authors approved the final version of the article. All authors had unrestricted access to the data and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol and had final responsibility for the decision to submit for publication.
Funding
This pooled IPD analysis was funded by The National Key Research and Development Plan (2022YFC2503503), the Chinese Society of Cardiology (grant number CSCF 2019-A0003), and the Jiangsu Provincial & Nanjing Municipal Clinical Trial Project (grant number BE 2019615).
Conflict of interest statement
S.-L. Chen reports speaker fees from MicroPort, Boston Scientific, Medtronic, Sanofi, Amgen, Pulnovo Medical, and BrosMed; received grants from the National Scientific Foundation of China; and is the faculty of the Key Laboratory of Cooperative Innovational and Translational Center at Nanjing Medical University. J. Zhang received grants from the National Scientific Foundation of China. The other authors have no conflicts of interest to declare. The Guest Editor reports receiving fees from Terumo.

