Mitral annular calcification (MAC) is characterised by the deposition of calcium salts in the fibrous mitral valve annulus, altering its structural integrity and function, and posing significant clinical implications. It predominantly affects the posterior aspect of the annulus, with extension into the posterior leaflet; in more severe instances, it can also present anteriorly1. MAC was originally considered a local, chronic, degenerative process but is now regarded as an active and regulated molecular process of injury and inflammation. Through various metabolic, inflammatory and mechanical factors, progressive fibrosis and calcification of the annular tissue are caused.
There are several risk factors that contribute to the development of MAC, including but not limited to female sex, advanced age, systemic hypertension, hypercholesterolaemia, diabetes mellitus, chronic renal failure, mitral valve prolapse and genetic abnormalities of the fibrous skeleton (e.g., Marfan and Hurler syndromes)23. Furthermore, recent studies have elaborated on risk factors that indicate progression of MAC and could be associated with poor outcomes or all-cause mortality. Lee et al, in a retrospective study including 560 patients with MAC, showed that MAC progression was independently associated with left ventricular ejection fraction, pulse pressure, MAC angle and transmitral mean diastolic pressure gradient (MDPG), whilst progressive disease was also linked with poorer clinical outcomes (a composite of heart failure hospitalisations, stroke and mortality)4. Transmitral MDPG has also been shown to be predictive of all-cause mortality, irrespective of the grade of mitral valve disease (MVD) severity, contrary to mitral regurgitation (MR) severity5. Besides echocardiographic variables, female sex is another predictor of adverse events, MAC progression to MR and mortality67. More recently, the role of lipoprotein(a) has been investigated as a potential pathophysiological indicator and promising disease predictor8. Understanding more about predictive factors in the near future and studying the pathogenesis of MAC is vital for early detection, enhanced risk stratification and prevention of potential complications, such as MR, mitral stenosis (MS), arrhythmias, infective endocarditis and thromboembolic events9.
Epidemiology and prognosis of MAC
The prevalence of MAC and related valvular disease increases with advancing age. Depending on the imaging modality used for MAC detection and the age subgrouping of the sample of each study, MAC prevalence rates vary from 5% to 10% in those aged over 60 years, and 33% to 42% in those aged over 80 years10111213. MAC tends to be more commonly detected in females than in males1415. Although it is considered a disease of the elderly, it can also affect younger patients who have comorbidities such as chronic kidney disease and metabolic disorders. When it comes to the prevalence of MAC in different racial groups, the data are limited and conflicting.
Two population-based studies pointed out that 2.2% and 6.6% of individuals had MAC-associated MS, and those numbers rise to 11.9% and 9.5% for significant MAC-related MR. Within the cohort of patients with MVD, those with MAC-related MVD have the poorest survival rates1516. Several studies have been investigating the association of MAC and cardiovascular disease (CVD), and there is strong evidence for a positive correlation. However, we have no indication that managing known risk factors has an impact on the progression of MAC. More specifically, the Framingham Heart Study demonstrates the independent association of MAC with incident CVD and CVD death, highlighting that cardiac calcification is a marker of increased CVD risk. In terms of numbers, for each 1 mm increase in MAC, the risk of CVD, CVD death, and all-cause death increases by approximately 10%10. Similarly, the Belgrade Atrial Fibrillation Study confirmed the association between MAC and all-cause death, CVD death, ischaemic stroke, and myocardial infarction17, while the Northern Manhattan Study demonstrated an association with myocardial infarction and vascular death, but not with ischaemic stroke18.
Diagnostic challenges: how to diagnose severe valvular disease in MAC
In the diagnosis of MAC, multiple imaging modalities can be used. Firstly, echocardiography, particularly two-dimensional transthoracic echocardiography (TTE), reveals MAC as an echodense, irregular structure, with calcifications most frequently located in the posterior annulus, producing acoustic shadowing1920. Given that mitral annulus fibrotic changes may mimic MAC, due to similar brightness and echodensity, it becomes clear that TTE has a limited ability to differentiate fibrosis from calcification, ultimately leading to overestimation of MAC severity compared with cardiac computed tomography (CT)19.
Transoesophageal echocardiography (TOE) offers a more enhanced image, providing detailed information on the anatomy of MAC, specifically, its location and leaflet movement, as well as qualitative stenosis and regurgitation severity, which can be further assessed by colour Doppler echocardiography1921. TOE imaging can also prove useful in differentiating MAC from other pathologies, such as tumour, thrombus, and infection19.
Multidetector CT is the preferred imaging modality for evaluating MAC. Due to its high spatial resolution and its ability to differentiate calcium from fibrosis, more precise mitral annular measurements and quantification of MAC can be achieved. This can be especially helpful in preparation for surgical or transcatheter interventions202122. Furthermore, it allows the calculation of the cardiac CT-based MAC score, which takes into account average annular calcium thickness (<5 mm: 1 point; 5-9.9 mm: 2 points; ≥10 mm: 3 points), calcium distribution in the annular circumference (<180°: 1 point; 180-270°: 2 points; >270°: 3 points), trigone calcification (none: 0 points; anterolateral: 1 point; posteromedial: 1 point), and mitral leaflet calcification (none: 0 points; anterior: 1 point; posterior: 1 point). MAC severity is then divided into mild MAC (≤3 points), moderate MAC (4-6 points), and severe MAC (≥7 points)19. Another quantitative method includes measuring the mitral calcium volume and Agatston score, which is adapted from coronary artery calcium scoring, in an attempt to provide a more reliable and subjective evaluation of MAC severity. A recent study by Eberhard et al evaluating this quantitative method in elderly patients with MAC showed that semiquantitative assessment of MAC had high interobserver agreement both in the absence of MAC and in the presence of severe MAC, but not in mild or moderate disease. On the contrary, minor inconsistencies were found when using the Agatston score23. Therefore, quantitative MAC assessment might be essential prior to transcatheter mitral valve replacement (TMVR) for accurately identifying the extent and severity of MAC, along with enhancing procedural planning in order to evaluate TMVR-induced left ventricular outflow tract (LVOT) obstruction22.
Cardiac magnetic resonance (CMR) has limited diagnostic value. CMR can be helpful in evaluating caseous calcifications, which typically present as a hypointense rim and a hyperintense centre on T1-weighted images, and vice versa on T2-weighted images, and in accurately quantifying the size of the chambers of the heart, their function, flow, and the severity of MR19. Nuclear imaging also offers minimal utility, whilst stress echo or invasive haemodynamic testing might be helpful in selected individuals with comorbidities21.
In summary, a multimodality imaging approach is essential for the accurate diagnosis, classification, and management of patients with MAC. Despite no universally accepted definition or classification system existing in societal guidelines, echocardiography serves as a first-line imaging modality which assists in initially diagnosing MAC and evaluating its relationship with adjunct structures. Advanced imaging with CT could help further analyse anatomical relationships, the extent of calcification, quantification of MAC severity and LVOT expansion/myocardial infiltration, enabling better identification of patient risk for mitral intervention and optimal preprocedural planning, which could lead to improved patient outcomes192022. However, whether an advanced imaging assessment of MAC in trial settings reduces procedure-related adverse events is still an unanswered question, in need of further research.
MAC and aortic stenosis: a pitfall in TAVI success
Several studies have revealed a significant overlap between MAC and aortic stenosis (AS), with MAC often present in patients diagnosed with calcific AS. The observation of this comorbidity suggests that MAC and AS may share some underlying pathogenic mechanisms24.
This coexistence poses challenges in the management of AS, particularly via transcatheter aortic valve intervention (TAVI). According to a meta-analysis published in April 2024, in patients with severe AS who underwent TAVI, the prevalence of MAC, severe MAC, and MAC-related MVD was 43%, 10%, and 6.8%, respectively25. MAC also challenges the optimal positioning and deployment of the transcatheter valve, increasing the chances for paravalvular leak (PVL) and conduction disturbances26.
For TAVI candidates with MAC, the progression rate of AS and MS depends on both the degree of MAC expansion and the thickness of MAC, indicating that they are potential risk factors for subsequent worsening of the stenosis27. However, the mitral calcium volume does not alter clinical outcomes after TAVI28. In addition, severe MAC is correlated with an increased incidence of major bleeding complications25. Nevertheless, it is not the MAC but rather the presence of concurrent MAC-related MVD which amplifies 30-day and 1-year mortality29.
Despite the anticipation that MR would improve after TAVI, as a result of reduced left ventricular pressure, MAC-related MR is unlikely to ameliorate following TAVI30, with a greater increase in postprocedural mitral gradients in patients with more severe MAC31. Consequently, the lack of improvement in MR worsens outcomes post-TAVI. In these cases, the severity of MAC should be considered when planning potential subsequent mitral valve interventions31. Thus, vigilant preprocedural assessment and tailored approaches, which include assessing the timing of intervention and deciding the type of intervention (isolated or concomitant), are vital in patients with challenging multivalvular disease undergoing TAVI in order to optimise procedural success, reduce surgical risk and improve clinical outcomes. In the context of transcatheter valve interventions, one of the latest innovations seen in several case reports has been to address both valves simultaneously3233. Advantages of concomitant interventions for both aortic stenosis and MAC-related mitral disease include reducing the adverse events observed post-TAVI in patients with residual MR by addressing both pathologies at the same time, especially in patients with MAC who, as noted, are not expected to improve regarding MR after TAVI, as well as decreased reinterventions and hospital costs. However, potential additional benefits, as well as the risks of combined interventions, need to be further investigated in future studies.
Management strategies: address your fears
There are two main strategies for treating mitral valve dysfunction accompanying MAC: surgical or transcatheter intervention (Figure 1). When the surgical risk is not prohibitive and the anatomy is permissive, conventional surgery remains the gold standard. Specific data are limited, but surgical mitral valve repair is the preferred option, since there is no substantial evidence in favour of surgical mitral valve replacement34.
TMVR is a promising alternative for MAC treatment. According to the device type, TMVR is categorised into TMVR with transcatheter aortic valves (TAVs) and TMVR with dedicated devices. TMVR using TAVs demands a rigid foundation to secure the valve in place; thus, it is the ideal option for patients with a failing bioprosthesis, surgical rings, or MVD with severe MAC. TMVR with dedicated devices aims to treat native MR, with or without MAC, offering an alternative to transcatheter edge-to-edge repair (TEER) when TEER is either not feasible or unlikely to produce satisfactory outcomes35. When severe calcification extends beyond the anterior commissure and invades the aortic mitral curtain, a transcatheter approach may be more appropriate as the surgical complexity increases significantly3637.
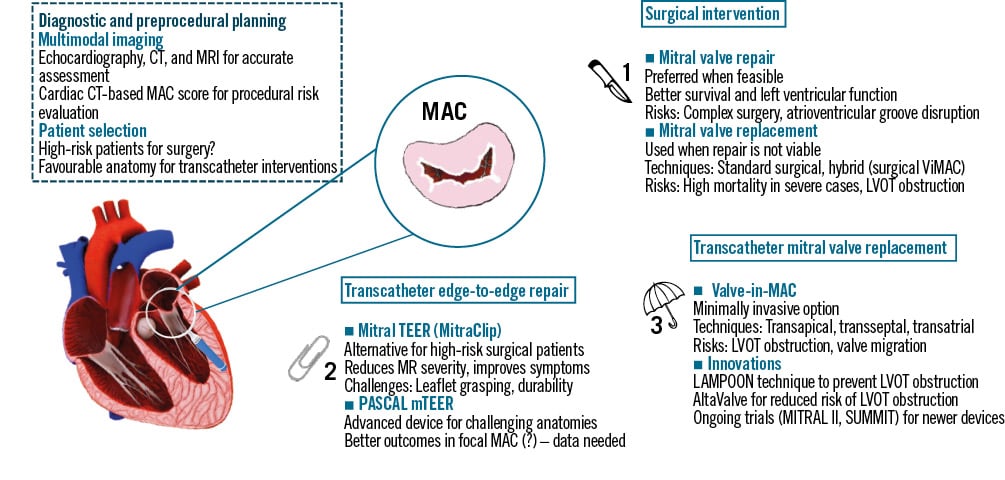
Figure 1. Management strategies for mitral annular calcification. MitraClip by Abbott; AltaValve by 4C Medical Technologies; PASCAL by Edwards Lifesciences. CT: computed tomography; LAMPOON: Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction; LVOT: left ventricular outflow tract; MAC: mitral annular calcification; MR: mitral regurgitation; MRI: magnetic resonance imaging; mTEER: mitral TEER; TEER: transcatheter edge-to-edge repair; ViMAC: valve-in-MAC
Surgical intervention
The presence of MAC is surgically challenging, and patients with MAC are defined per se at high surgical risk, given their higher rates of conduction disturbances, circumflex artery rupture, and embolism post-surgery. Interestingly, a study that used the Society of Thoracic Surgeons risk score to identify potential differences in mortality between patients with MAC undergoing surgery found that the presence of MAC independently increases operative risk in all risk categories38. Moreover, patients with MAC-related MVD are typically old with multiple comorbidities. A medical history of mediastinal radiation (particularly affecting the anterior annulus and intervalvular fibrosa) and chronic kidney disease, which is strongly associated with MAC, independently increases the risk of cardiovascular death after cardiac interventions39.
As there is no designated score, to date, for estimating the surgical risk of patients with MAC-related mitral valve disease that takes into consideration traditional surgical risk factors, baseline characteristics, and preprocedural imaging parameters, the Heart Team should aim to discuss and assess the overall risk as well as the risk for complications, such as patient-prosthesis mismatch, PVL (common in large annuli), conduction disturbances, and rupture of the atrioventricular groove. Furthermore, the extension of MAC into the left heart cavities (atrium or ventricle) may change the surgical plan. Especially in cases where MAC extends into the LVOT, subvalvular resection or septal myectomy are often performed simultaneously in order to alleviate LVOT obstruction and improve haemodynamics.
Surgical outcomes are statistically inferior in patients with MAC, compared to those without significant MAC. A study by Kaneko et al on patients undergoing surgical mitral valve replacement (SMVR) showed a higher estimated inpatient mortality in patients with MAC (5.8%), compared to patients without (4.4%)38. However, a systematic review of 15 surgical studies reported an exceptionally wide range of mortality at 30 days, 1 year, and 5 years. These variances can be attributed to the various surgical and anatomical risks of the individuals included in each study. These risks are present in patients with MAC but may have been underreported in the studies included in this analysis40. Despite the above, conventional SMVR is still the preferred intervention and can lead to favourable outcomes provided patients overcome the increased initial risk.
In general, mild MAC of the posterior annulus, involving less than one-third of the annular circumference, does not affect surgical valve intervention using conventional techniques. The surgical management of moderate or severe MAC can be carried out either through an extensive en bloc resection with annular reconstruction – the “resect” method – as well as more “respectful” alternatives, targeted conservative decalcification, or no resection at all. Each technique has its own merits and demerits34. The “respect” technique allows prosthetic valve implantation on top of the calcium bar, without requiring its removal, but it can result in poor sealing and significant PVL. On the other hand, the “resect” technique, which is suitable for larger prostheses and provides better sealing with reduced PVL, carries the risk of weakening the mitral annulus and is associated with higher rates of atrioventricular groove dissociation, left ventricle perforation, and injury to the left circumflex artery. Moreover, the “resect” technique is more complex, requiring longer crossâclamp and cardiopulmonary bypass times, and thus presents higher mortality rates41.
The limited data consistently show mitral valve repair as superior to replacement when it comes to survival, complication rates and post-surgery left ventricular function. Even so, the frequency of conversion from repair to replacement is higher in MAC patients compared to other cases (8% vs 3%)34. Mitral valve replacement in the setting of severe MAC has been associated with a high risk of left ventricular aneurysm and rupture, as well as acute posterior myocardial infarction. Standard surgical valve replacement techniques can be used in patients with MAC when repair is not viable. It is preferred to preserve the subvalvular apparatus, as the risk of midventricular tears increases otherwise. The annular sutures can be secured around the calcium bar, through the leaflets, or both at the same time.
Direct transatrial transcatheter mitral valve replacement
Surgical valve-in-MAC (ViMAC), i.e., insertion of a balloon-expandable transcatheter heart valve via left atriotomy and deployment under direct vision, can avoid the need for annular decalcification by creating a larger effective orifice area, compared with a surgical prosthesis42. This hybrid approach is indicated for high-risk patients, for whom other conventional approaches are unsuitable, or patients who are at risk for LVOT obstruction with TMVR. Small case series have demonstrated good echocardiographic results and improvement of symptoms but high inpatient and 30-day mortality43. Although this approach appears to be an appealing alternative in high-risk and comorbid patients in terms of echocardiographic/functional outcomes, small retrospective studies also show a 1-year mortality rate >30%44. Patients with significant MR may derive less benefit from ViMAC than patients with less severe MR45]. To address the gaps in knowledge and investigate mortality rates and adverse outcomes, the Surgical Implantation of Transcatheter Valve in Native MAC (SITRAL) study (ClinicalTrials.gov: NCT02830204) (Table 1) is an ongoing study assessing the safety and effectiveness of surgically implanting bioprosthetic valves (SAPIEN 3 [Edwards Lifesciences]) in MAC patients who present with high operative risk due to the extent of their calcification. The study will provide comprehensive 30-day and 1-year outcomes.
Table 1. Completed clinical trials.
| Clinical trial identifier |
Valve/ intervention | Population | Number of patients | Completion date | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| SITRAL (NCT02830204) |
Mitral valve replacement with SAPIEN 3a | MAC with MS/MR, at high risk for mitral valve surgery, or inoperable because of the extent of calcification, NYHA Class ≥II, age ≥22 y.o. | 25 | September 2023 | Not published | |||
| Procedural (at 30 days), | ||||||||
| technical (at procedure stop time), | ||||||||
| and device (at 30 days) | ||||||||
| success | ||||||||
| MITRAL (NCT02370511) |
Mitral valve replacement with SAPIEN XTa/SAPIEN 3a | MAC with severe calcific native MR (MVA ≤1.5 cm2) or severe MR and at least moderate MS, NYHA Class ≥II, age ≥22 y.o. 3 groups: native mitral valve with severe MAC (ViMAC), ViR, ViV |
91 | December 2018 | Sustained improvement of heart failure symptoms and quality of life among survivors at 5 years | |||
| ViMAC | ViR | ViV | ||||||
| Technical success, % | 74.2 | 66.7 | 100 | |||||
| Absence of MR grade 2+ or greater | 92/ | 100/ 100 | 100/ 100 | |||||
| (% at 30 d/1 y) | 100 | |||||||
| Median MVG (mmHg at | 6.0/ 6.1/ 6.7 | 7.6/ 6.0/ 5.8 | 6.0/ 6.6/ 6.6 | |||||
| 30 d/1 y/5 y) | ||||||||
| 30-day procedural success, % | 53.3 | 73.3 | 93.3 | |||||
| 5-year all-cause mortality, % | 67.9 | 65.5 | 21.4 | |||||
| 5-year NYHA I or II, % | 55.6 | 50 | 94.7 | |||||
| aBy Edwards Lifesciences. MAC: mitral annular calcification; MR: mitral regurgitation; MS: mitral stenosis; MVA: mitral valve area; MVG: mitral valve gradient; NYHA: New York Heart Association; ViMAC: valve-in-MAC; ViR: valve-in-ring; ViV: valve-in-valve; y.o.: years old | ||||||||
Transcatheter edge-to-edge repair
Treating MR in the presence of MAC is, to this day, a challenge due to the risks associated with surgical approaches. Mitral TEER has emerged as an alternative therapy that reduces MR severity, reverses left ventricular remodelling and provides symptom relief. There is, however, limited evidence regarding its feasibility and durability, as well as its prognostic value in MAC, since patients with MAC were excluded from large clinical trials21. Interestingly, a study by Tanaka et al46, evaluating the role of CT-based assessment of MAC in patients undergoing TEER, showed that higher mitral valve calcium volume and MAC score were inversely related to procedural success, while both were, independently of baseline and procedural factors, associated with a higher risk of all-cause mortality. Moreover, despite the results from the available studies showing a positive outcome, a potential selection bias applies to these studies, given their observational and retrospective nature.
Until recently, TEER was not considered a good option for such patient phenotypes, considering the progressive calcification of the valvular leaflets, which might progress to device-related stenosis19. Nonetheless, recent studies indicate that moderateâtoâsevere MAC was not associated with decreased technical success47. According to one study, there were no significant differences between patients with MAC and patients with none/mild MAC in terms of procedural success (88.5% vs 94.9%; p=0.12), MR reduction (1-month residual MR ≤2 in 92.9% of nonâMAC patients and 91.6% of MAC patients) or New York Heart Association (NYHA) Class improvement. At 1-year followâup, the need for further MR reintervention remained low in the moderate-to-severe MAC population (6.5%) and was comparable to the none/mild MAC group (2.8%; p=0.26). Clinical outcomes following TEER in MAC patients also show significant improvements in MR severity and NYHA Functional Class4849. Another study highlighted that TEER can be performed effectively in selected patients with severe MAC, especially when calcification is focal and allows for adequate leaflet grasping48.
Despite the encouraging results, there was a difference in the 1-year mortality, with MAC being related to higher mortality percentages. Ît should be noted, however, that patients with MAC have a larger number of comorbidities and are, in general, at higher surgical risk, which could explain this finding47. Moreover, there are some complications of TEER in MAC that are not insignificant, including the retraction of the posterior valve leaflet, the extension of calcium onto the leaflets, or the small area of the native valve. All of the above minimise the amount of tissue that is available to grasp48. Conversion to surgery was more frequent in MAC patients, consistent with former studies and likely reflecting the higher procedural complexity50. Durability appears to be decreased in patients with severe MAC, even though, at 1-year follow-up, the durability of the repair in selected patients with moderate or severe MAC was similar to that in those without MAC51. It should also be noted that, given the lack of more robust, long-term clinical results in this patient cohort, there is a possibility that MR reduction may not ultimately improve such outcomes. Further clinical trials are necessary in order to fully investigate TEER in MAC, while newer devices, such as the PASCAL system (Edwards Lifesciences), may provide further options in patients with MAC, as they offer different manufacturing components from MitraClip (Abbott) and, therefore, could be of use in challenging anatomies where MitraClip use could be suboptimal50. However, such scenarios should be formally investigated in clinical studies, as MitraClip and PASCAL have comparable results in both the short and long term52.
Transcatheter mitral valve replacement
TMVR, and particularly ViMAC, a minimally invasive procedure where balloon-expandable valves are placed via a catheter, has shown encouraging results in preliminary studies53. It can be carried out via a transapical, transseptal or transatrial access, with the transfemoral transseptal access being the most used. Interestingly, studies have shown that transseptal delivery of the valve has a survival benefit over transapical access54. Advantages of the transapical method include an excellent coaxial alignment of the prosthetic valve, which can help to improve procedure- and device-related adverse events, such as PVL55. However, it requires a thoracotomy and therefore is more invasive, thus explaining the aforementioned benefit of transseptal techniques. On the other hand, transseptal delivery is less invasive and also has a feasible coaxial alignment. However, it presents more challenges in device development and could potentially promote iatrogenic atrial septal defects. The field of TMVR, especially considering novel devices, is expanding, with several valves being investigated that are delivered either transapically (Intrepid [Medtronic], Tendyne [Abbott]) or transseptally (Cardiovalve [Venus Medtech], AltaValve [4C Medical Technologies], Cephea [Abbott], Evoque [Edwards Lifesciences]). Recent studies have reported on the safety and feasibility of such valves, showing promising results5657. In this context, several investigators have aimed to evaluate the safety and efficacy of TMVR in patients with MAC.
In terms of clinical outcomes, TMVR has so far proved to be safe and efficient. In their study, Eleid et al reported a 1-year survival rate of 57%, with symptom amelioration in patients with severe MAC undergoing TMVR58. The MAC Global Registry found a technical success rate of 76.7% in TMVR procedures but noted a 1-year mortality rate of 53.7%, primarily due to severe LVOT obstruction. Additionally, the MITRAL trial (ClinicalTrials.gov: NCT02370511) similarly reported a technical success rate of 74.2% for ViMAC procedures, with a 1-year mortality rate of 34.5%58, while Praz et al documented a 100% technical success rate for transatrial TMVR, with a 30-day mortality rate of 27%43. Moreover, early results from Gössl et al, including 20 patients with MAC and using the transapical Tendyne system59, reported no periprocedural mortality, with 30-day and 1-year mortality being 5% and 40%, respectively. No valve dysfunction was noted, while clinical and functional improvements were noted in the majority of patients who were alive at the last follow-up.
Despite these promising results, TMVR in MAC patients poses several challenges and potential complications. One of the most significant concerns is LVOT obstruction, which, despite being relatively infrequent, can lead to severe haemodynamic compromise and increased mortality. In cases of LVOT obstruction risk, techniques such as the Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction (LAMPOON) have been developed to address this issue by creating a larger outflow tract and reducing the risk of obstruction5360. Alternative methods include perioperative alcohol septal ablation in selective cases and surgical anterior leaflet resection prior to the transcatheter MV replacement in cases with a transatrial approach. The efficacy of LAMPOON and surgical leaflet resection are being prospectively studied in clinical trials (ClinicalTrials.gov: LAMPOON trial: NCT03015194; and SITRAL trial: NCT02830204)61. Furthermore, newer leaflet modification techniques could be useful in preventing LVOT obstruction, including the ShortCut device (Pi-Cardia), which is being used for leaflet modification in the aortic valve and has shown positive results in preventing coronary artery obstruction62. Transseptal Balloon-Assisted Translocation of the Mitral Anterior Leaflet (BATMAN) is another promising alternative for LVOT obstruction prevention. It mimics the surgical posterior translocation of the anterior leaflet and could be a valuable technique for transcatheter interventions63. Finally, the AltaValve has been developed with the consideration of LVOT obstruction in mind. It stands out with its unique design and supra-annular placement, positioned exclusively on the atrial side of the mitral valve, and is currently being evaluated (AltaValve Early Feasibility Study Protocol; NCT03997305) (Table 2)35.
Another challenge is the risk of valve migration and embolisation. Periprocedural risk is particularly high in ViMAC procedures, where the uneven geometry of a heavily calcified mitral annulus can complicate annular sizing. In contrast to valve-in-valve TMVR, where the prior surgical valve provides excellent and predictable anchoring, MAC unpredictably changes annular pliability during balloon expansion and increases variability in final positioning. Recently, a CT score was developed to grade MAC severity and predict the possibility of transcatheter heart valve migration or embolisation. Patients with MAC scores of 6 or less have a very high risk for device migration or embolisation compared with patients with MAC scores of 7 or greater (60.0% vs 9.7%). Using cardiac CT imaging in the evaluation of patients with MAC, as well as adequate oversizing, has resulted in an embolisation rate of zero in the prospective MITRAL trial (ClinicalTrials.gov: NCT02370511)19.
Although clinical outcomes after TMVR have improved over the years, mortality after ViMAC remains considerable, and further refinements in patient selection and procedural planning are needed60. Imaging techniques, such as CT and echocardiography, play a vital role in ensuring appropriate device size and placement. Future developments, including specifically designed TMVR devices that may offer enhanced stability and reduced complications compared to current devices, are an active research frontier and may also make it possible to treat a wide range of anatomies3553.
Concerning the comparison between TMVR and TEER, it must be noted that TMVR offers several advantages that outweigh TEER’s limitations. Firstly, in terms of MR reduction, TMVR accomplishes complete resolution, whereas TEER often results in residual MR. TMVR may also be superior regarding patient suitability. Specifically, only the transcatheter replacement method can be used in patients with severe MAC, as well as in patients with valve-in-valve and valve-in-ring, while TMVR has the potential to be used for mitral stenosis, for which there is no such indication for transcatheter repair35.
In conclusion, TMVR offers a promising alternative for high-risk patients with MAC, presenting a less invasive option compared with traditional surgery. A multidisciplinary approach is essential for optimising patient selection and procedural success. Continued innovation, collaborative care, and comprehensive research are necessary to achieve the best possible outcomes for these patients, addressing current fears and enhancing the viability of TMVR as a mainstream treatment for MAC53.
Table 2. Ongoing clinical trials.
| Clinical trial identifier |
Valve/ intervention | Population | Number of patients | Completion date | Outcomes |
|---|---|---|---|---|---|
| AltaValve Early Feasibility Study Protocol | AltaValvea TMVR | Age ≥18 y.o., symptomatic NYHA II-IV, severe MR, subjects who are at high risk for open-heart surgery | 15 | September 2025 | Major adverse cardiac events (cardiac death, stroke, mitral valve-related repeated intervention) (at 30 days) |
| (NCT03997305) | |||||
| MITRAL II | Transseptal ViMAC | Age ≥18 y.o., severe MAC with severe MS, or ≥moderate to severe MR, or mixed ≥moderate MS and ≥moderate MR, NYHA Class ≥II, at high risk for standard surgery | 210 | December 2024 | A non-hierarchical composite of all-cause mortality and hospitalisation for heart failure (at 1 year) |
| (NCT04408430) | |||||
| SUMMIT – MAC Cohort | Tendyneb mitral valve system, MitraClipb System | Symptomatic, moderate-to-severe or severe MR, or severe MAC, NYHA Class ≥II | 958 | June 2028 | Survival free of heart failure hospitalisation at 12 months post-index procedure |
| (NCT03433274) | |||||
| Feasibility Study of the Tendyne Mitral Valve System in MAC | Tendyneb mitral valve system | Symptomatic and severe MR, NYHA Class ≥II, age ≥18 y.o., not suitable for conventional surgical treatment | 11 | November 2024 | Device success and freedom from device- and procedure-related serious adverse events per MVARC criteria (at 30 days) |
| (NCT03539458) | |||||
| aBy 4C Medical Technologies; bby Abbott. MAC: mitral annular calcification; MR: mitral regurgitation; MS: mitral stenosis; MVARC: Mitral Valve Academic Research Consortium; NYHA: New York Heart Association; TMVR: transcatheter mitral valve replacement; ViMAC: valve-in-MAC; y.o.: years old | |||||
The future is now
Taking into consideration the exponential advancement of methods used for the management of MAC, there are several promising further developments. At the time of writing, there are two ongoing trials aiming to evaluate the effectiveness and safety of new valves used in replacement management. The MITRAL II Pivotal Trial (ClinicalTrials.gov: NCT04408430) focuses on reviewing the SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA valves (all Edwards Lifesciences) in patients with severe MAC and symptomatic mitral valve dysfunction who are not candidates for standard mitral valve surgery, with estimated study completion in December 2024. The Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Transcatheter Mitral Valve System for the Treatment of Symptomatic MR (SUMMIT; NCT03433274), specifically its non-randomised MAC cohort, aims to assess the Tendyne transcatheter mitral valve system for the treatment of patients with symptomatic mitral valve disease due to severe MAC. Also ongoing, with an estimated study completion date in June 2028, this randomised controlled trial will provide a comparison to the MitraClip System in patients with symptomatic, moderate-to-severe or severe MR.
Conclusions
In conclusion, MAC is a challenging condition that requires a comprehensive understanding of pathophysiology, diagnosis and therapeutic alternatives. The evolving quiver of management strategies, including novel techniques like direct transatrial TMVR and TEER, promises alternative options for MAC patients deemed high risk for surgery. In the near future, promising results are anticipated from ongoing clinical trials, as well as technological developments that will further enhance patient outcomes and redefine treatment approaches in patients with MAC.
Conflict of interest statement
The authors have no conflicts of interest to declare.

