Introduction
The complexity of percutaneous coronary interventions (PCI) is rising, and with it, the indication for percutaneous revascularisation of complex anatomical subsets is expanding12. Patients with unprotected left main (UPLM) disease, multivessel disease (MVD), chronic total occlusions (CTO), severely depressed left ventricular function and those who are turned down for surgical revascularisation (collectively termed “high-risk PCI patients”) are inherently at an elevated risk of periprocedural complications due to the complexity of their coronary disease and medical comorbidities. These patients are now being offered PCI, whereas previously they were associated with a “risk-treatment paradox” in which they were least likely to be offered revascularisation despite carrying strong indications for this therapy34567. The number of high-risk patients undergoing PCI (high-risk PCI) has increased in the last few years, however, and physiological and haemodynamic factors in combination with procedural difficulties pose unique challenges that influence clinical outcomes28.
In India and South East Asia, multiple diagnostic and therapeutic technologies have been introduced that are aimed at facilitating revascularisation in complex and high-risk patient subsets. However, use of the Impella percutaneous left ventricular assist device (pLVAD; Abiomed) as a strategy to reduce the risk of haemodynamic instability during these high-risk procedures is limited. There is a lack of regional consensus on, firstly, what constitutes “high-risk PCI” and, secondly, on the guiding principles for patient selection for such devices. The decision-making process gets clouded further by the additional financial implications in an economically constrained health care environment, such as in India. This led to the creation of the India Working Group (IWG) on high-risk PCI under the guidance and endorsement of the Indian Foundation of Advanced Cardiovascular Interventions (IFACI: a foundation of pioneering interventional cardiologists in India which holds an annual Complex High-Risk PCI National Scientific Meeting). The IWG was tasked to construct an expert consensus document to guide the use of the Impella pLVAD in high-risk PCI. The IWG is comprised of nationally acclaimed interventional cardiologists who were recognised for their expertise and thought leadership in high-risk PCI. This working group met in person at a meeting held in January 2020 during the Second CHIP-CTO India Scientific Sessions, during which a review of their collective high-risk PCI experience and of key pLVAD data was conducted. Through a consensus discussion, considering various clinical high-risk PCI scenarios, an Impella-supported high-risk PCI patient selection tool was developed to guide its application in India.
This consensus document outlines the rationale, key data from the collective worldwide experience, and other considerations involved in creating a score to guide pLVAD support for high-risk PCI.
Patterns of cardiovascular disease in India
India, home to 18% of the world’s population, has undergone an enormous epidemiological transition which has resulted in massive variations in disease burden across the Indian states. This has led to an important rise in the prevalence and consequences of ischaemic heart disease (IHD). According to a comprehensive report by the India state-level disease burden initiative, deaths due to non-communicable diseases (NCDs) increased between 1990 and 2016; where, in 1990, the top 5 individual causes of disease burden were all communicable diseases, in 2016, 3 of the top 5 causes were NCDs, with IHD being the most common cause of disease burden9. As compared to 1990, in 2016, IHD alone was responsible for a 135% mean percentage change in the number of deaths, representing a 55% increase in the mean all-age death rate and a 34% increase in all-age disability-adjusted life years (DALYs)9.
With the rising prevalence of IHD in India, the concept of high-risk PCI becomes more important not only due to anatomical and pathological changes but also due to changes in risk factors and comorbidities (Figure 1) which may be the driver of the increasingly complex coronary artery disease (CAD) being encountered. For example, Indians over the age of 40 have a higher prevalence of multivessel involvement, diffuse disease, and arterial calcification10. In the coronary interventional registry of Indian patients undergoing coronary angiography and percutaneous coronary intervention in 2011, out of 193,728 stents used, nearly 30% of stents were deployed in the setting of multivessel CAD11. In corroboration with this, cross-sectional data from the National Interventional Council in 2018 also noted a rise in the rate of complex coronary interventions, with more patients undergoing left main and multivessel PCI in 2018, as compared to previous years12. Interestingly, similar observations have been noted amongst South Asians undergoing angiography in the United States, who have been found to have smaller coronary luminal diameters, higher-grade coronary artery obstructions and a higher prevalence of multivessel disease131415. These observations may point to a genetic component among Indians which predisposes them to multivessel CAD.
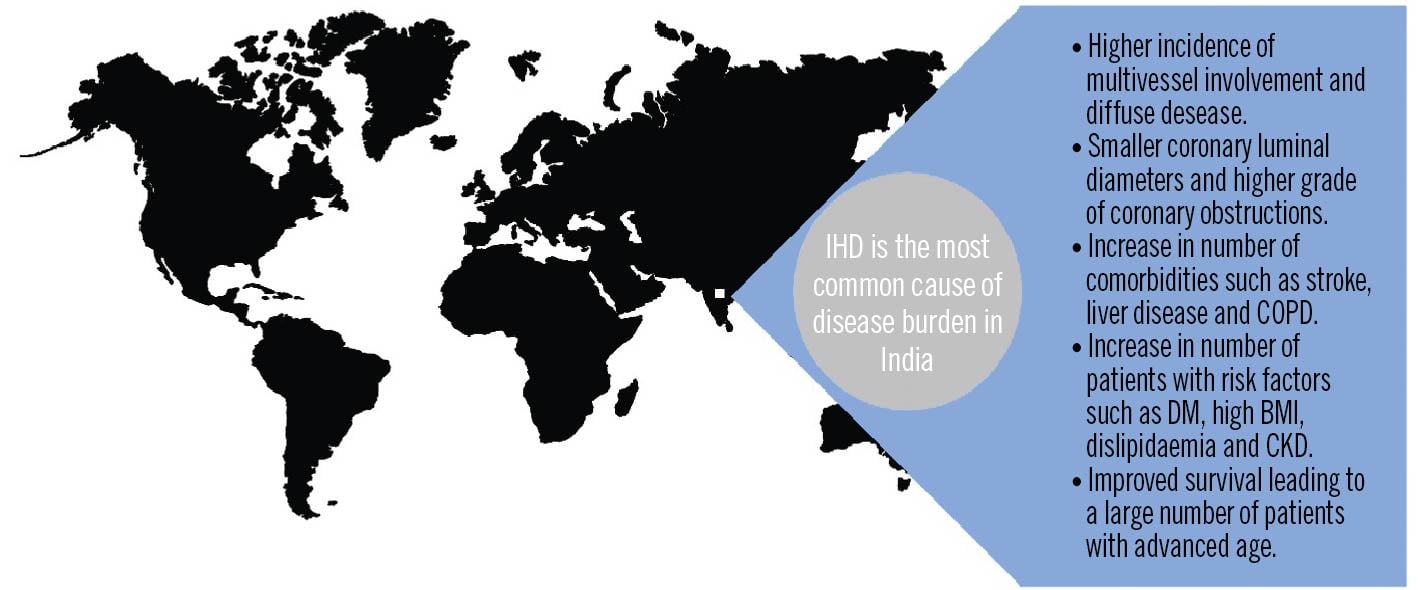
Figure 1. Risk factors contributing to high burden of complex coronary artery disease in India. BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; IHD: ischaemic heart disease. Created with BioRender.com
A rise in comorbidities that render patients at higher risk for surgical revascularisation, such as advanced liver disease, stroke and chronic obstructive pulmonary disease (COPD), has also been observed in India29. Similarly, increased age at the time of the intervention11, diabetes mellitus (DM), a high body mass index, dyslipidaemia, and chronic kidney disease may serve as drivers of complex CAD (e.g., chronic total occlusions)4 and are also increasing in the Indian population916. Compared to 1990, the all-age DALY rate among the Indian population in 2016 has increased by 150% for a high body mass index, by 80% for DM, 35% for patients with high cholesterol, 24% for patients with high blood pressure, 18% for alcohol use and 12% for patients with chronic kidney disease (Figure 2)9.
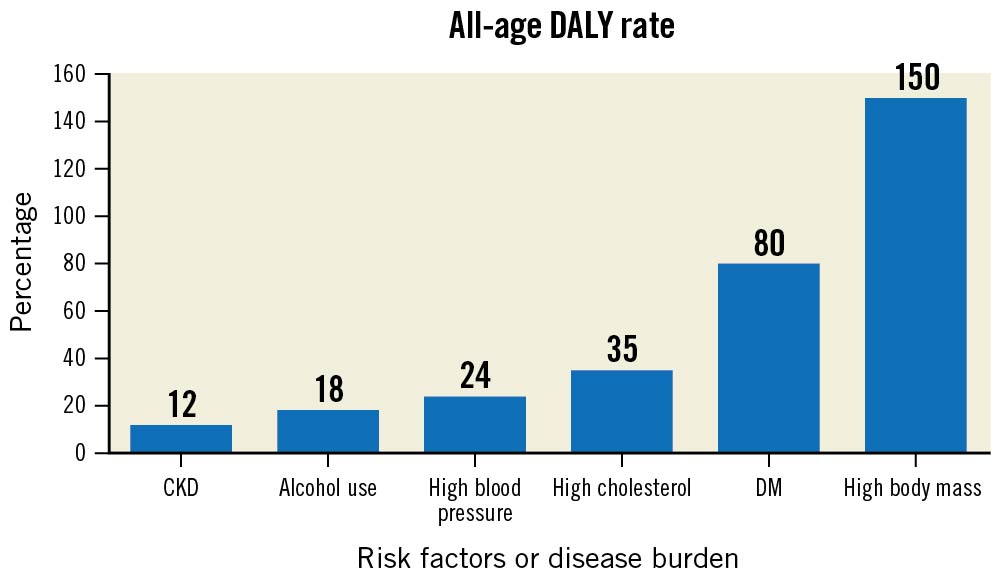
Figure 2. Trend of risk factors/disease burden in age-related disability adjusted life years (DALY) in India between 1990 and 2016. CKD: chronic kidney disease; DM: diabetes mellitus
A combination of the aforementioned factors has led to an increased number of high-risk patients among the Indian population who may be undergoing complex PCI and, therefore, highlights the importance of guidance for the use of pLVAD devices in these cases to improve outcomes.
clinical need and evidence for supporting devices during complex and high-risk PCI
Patients undergoing complex and high-risk PCI often require multiple and prolonged balloon inflations, repetitive contrast dye injections or aggressive lesion preparation with techniques such as atherectomy. These necessary steps, however, may result in transient myocardial ischaemia, which may manifest as ventricular systolic dysfunction517. This ventricular dysfunction further causes a rapid loss of arterial pressure, compromised coronary blood flow, decreased systemic perfusion and may continue to profound hypotension, leading to haemodynamic instability. These findings are translated to decreased mean arterial pressure (MAP), decreased cardiac power output (CPO) and increased left ventricular end diastolic pressure (LVEDP), which can lead to increased wall stress and increased myocardial oxygen demand1718. The upfront use of pLVAD protection devices for haemodynamic support has led to the maintenance of coronary blood supply and decreased myocardial oxygen demand (Figure 3), thereby facilitating more complete revascularisation and an overall decrease in short-term major adverse cardiovascular and cerebrovascular events (MACCE)19.
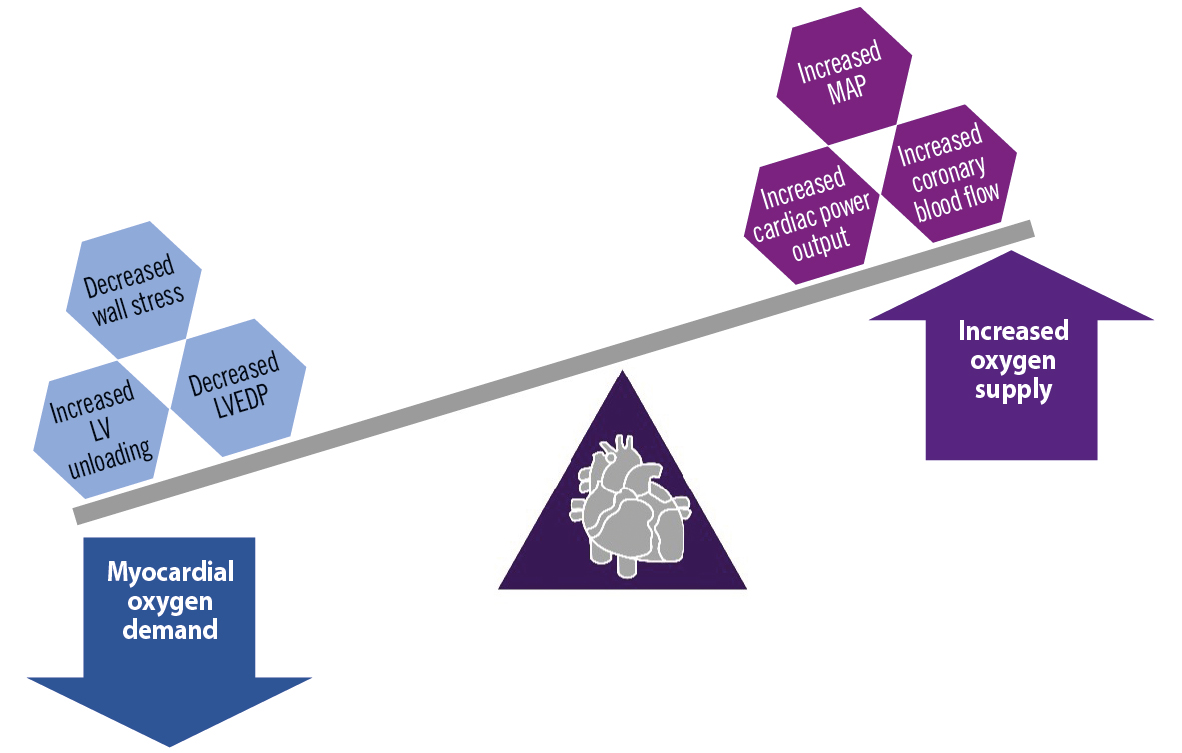
Figure 3. Physiological effects of percutaneous left ventricular assist devices on the myocardial oxygen demand and supply. LV: left ventricular; LVEDP: left ventricular end diastolic pressure; MAP: mean arterial pressure. Created with BioRender.com
Amongst the temporary haemodynamic support devices (pLVAD) currently available in India, the intra-aortic balloon pump (IABP) and the Impella pLVAD are the most commonly used. The Impella has recently been extensively studied with several completed and ongoing trials52021. The functioning of these devices and their respective impact on haemodynamics are presented in Figure 4. The IABP functions on the principle of counterpulsation and is dependent on an intact native cardiac function. It is capable of very modest left ventricular (LV) unloading through afterload reduction and increased coronary blood flow during augmentation. Conversely, the Impella pLVAD directly unloads the LV and actively propels blood from the LV into the aorta2223. The active forward flow leads to an effective maintenance of MAP and overall CPO and reduces LVEDP with concomitant LV unloading. Further, the Impella directly maintains coronary perfusion by propelling blood down the coronary arteries. The combination of these factors leads to a favourable alteration of the balance of myocardial oxygen supply and the demand for high-risk PCI patients supported with these devices18242526.
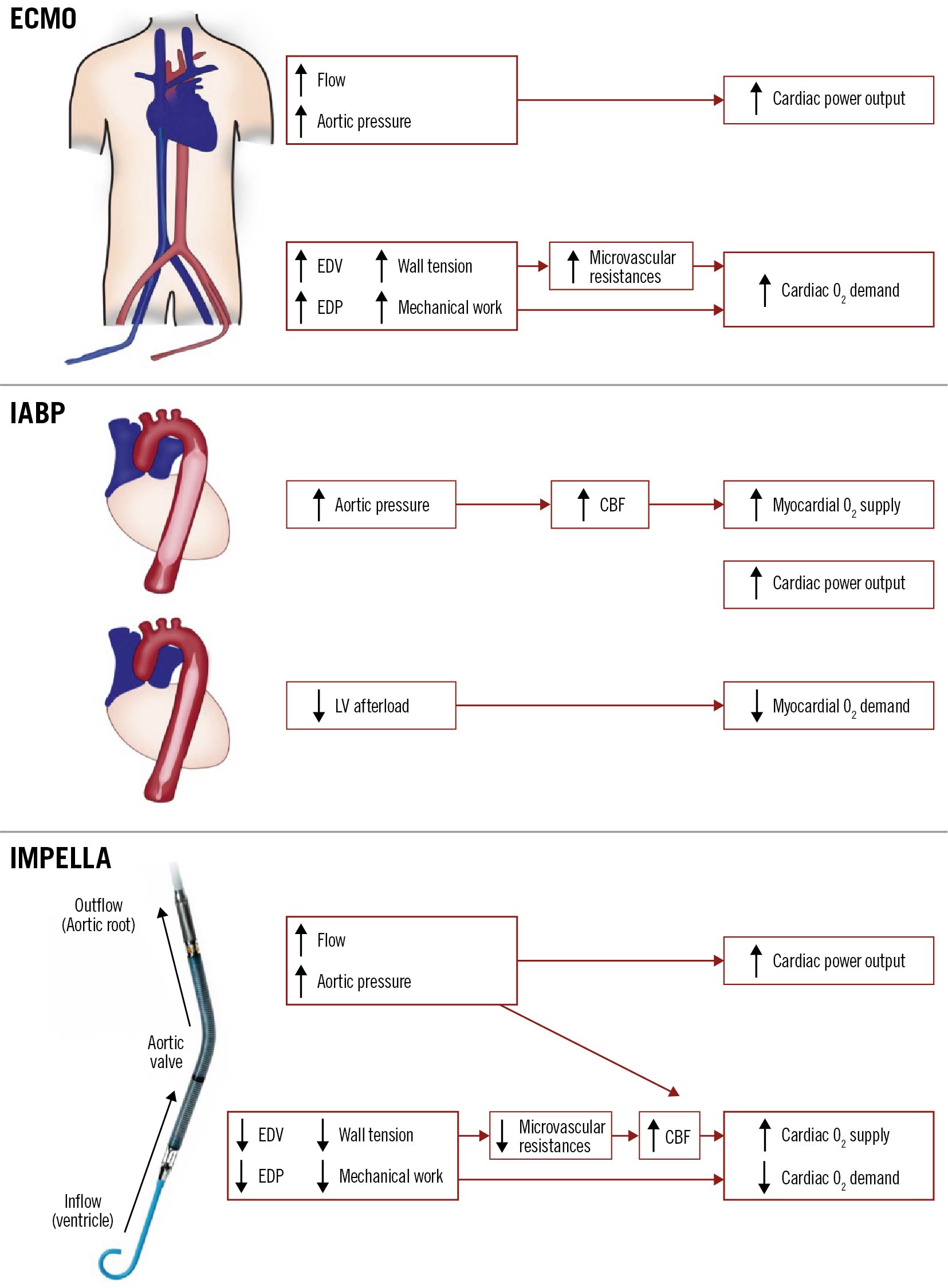
Figure 4. Graphical representation of haemodynamic effects of various percutaneous left ventricular assist devices. CBF: coronary blood flow; ECMO: extracorporeal membrane oxygenation; EDP: end diastolic pressure; EDV: end diastolic volume; IABP: intra-aortic balloon pump; LV: left ventricular; O2: oxygen
Evidence of safety and efficacy of supporting devices in complex and high-risk PCI
Data supporting the safety and effectiveness of the IABP and the Impella in haemodynamically supported PCI include several retrospective studies, randomised controlled trials (RCTs), registry studies and case reviews. The Balloon Pump-Assisted Coronary Intervention study (BCIS-1) was the first randomised controlled trial to assess the efficacy and safety of the elective use of IABP in patients undergoing high-risk PCI. In this trial, elective IABP insertion did not reduce the incidence of major adverse cardiac and cardiovascular events (MACCE; odds ratio [OR] 0.94, 95% confidence interval [CI]: 0.51-1.76; p=0.85)21. However, at a median of 51 months, the patients who received elective IABP during PCI had a 34% reduction in all-cause mortality compared to unsupported PCI20. This was followed by the Patients Undergoing High-Risk Percutaneous Coronary Intervention (PROTECT I) feasibility trial in which the Impella 2.5 device (Abiomed) was successfully implanted in all patients, and none of the patients developed haemodynamic compromise during PCI20. PROTECT II was a prospective, multicentre, randomised trial comparing outcomes between the Impella 2.5 and the IABP in patients with complex multivessel or unprotected left main CAD with concomitant severe left ventricular systolic dysfunction (LVSD) who required non-emergent high-risk PCI. The primary composite endpoint in this study of major adverse events (MAE) at hospital discharge or 30 days (whichever came sooner) was similar in both arms (IABP: 40% vs Impella: 35.1%; p=0.277)5. However, subsequent analysis of the PROTECT II data with a clinically relevant definition of periprocedural myocardial infarction showed that treatment with an Impella device was one of the independent predictors for lower MACCE and MAE at 90 days of follow-up27. The Impella also provided better intraprocedural haemodynamic support as compared to IABP. Further, at the 90-day study exit follow-up, there was an average 22% relative increase in left ventricular ejection fraction (LVEF) from baseline and a 58% improvement in New York Heart Association (NYHA) Functional Class III/IV5. Moreover, in patients undergoing extensive revascularisation, a significant reduction of MAE was seen in patients who received Impella support as compared to IABP support for their procedures (41.4% vs 54.0%; p=0.02)28. More recently, in another retrospective single-centre study of patients undergoing high-risk PCI, the use of the Impella for haemodynamic support was independently associated with a significant reduction in acute kidney injury (AKI) as compared to patients with no haemodynamic support (5.2% vs 27.8%; p<0.001)29. A consistent reduction in MACCE was also observed at 90 days in the PROTECT III trial, in the data presented at the transcatheter cardiovascular therapeutics conference19.
Outside of the RCT setting, Baumann et al, in their analysis of the German Impella registry, also showed acceptable in-hospital and 180-day clinical outcomes regarding major adverse cardiac events (MACE; 12.7% and 22.8%, respectively)30. Sjauw et al, in their analysis of the Europella registry, showed similar results, with MACCE being observed in 12.4% of cases at 30 days and, thus, supported the safety, feasibility and potential usefulness of haemodynamic support with the Impella31. The use of the Impella was also supported in the analysis of the USpella Registry, where sufficient haemodynamic support to facilitate high-risk single- or multivessel PCI led to an improvement in ejection fraction and an improvement of functional status. Moreover, the USpella Registry data also showed a low 30-day MACE rate of 8.2% and an 88% survival rate at 12 months32.
Of note, there are no RCTs comparing the Impella to extracorporeal membrane oxygenation (ECMO) or the TandemHeart (CardiacAssist) system (left atrial to aortic bypass) with regard to haemodynamic support in high-risk PCI. Notably, both of these support systems require very large-bore access and specialised implantation and management techniques (e.g., a transseptal puncture for the TandemHeart system, antegrade distal limb perfusion for placement of arterial ECMO cannulas due to larger diameter). Devices with simple and more commonly utilised implantation techniques, requiring smaller access sites that provide adequate haemodynamic support, such as the IABP or Impella, may be more desirable in regions where experience with haemodynamic support devices is nascent24. All available data have resulted in recommendations by experts and consensus statements supporting the use of haemodynamic support devices in patients undergoing high-risk PCI333435.
Defining the complex and high-risk PCI patient population
There is no universal definition of what constitutes “complex and high-risk PCI”, but several consensus documents3536 have provided guidelines to help identify patients who require these procedures. These guidelines also recommend the formation of multidisciplinary Heart Teams to guide appropriate patient selection, preprocedural assessment, treatment, and periprocedural care. Below, clinical characteristics identified by the IWG as germane to the designation of “high-risk PCI” are outlined and discussed.
Reduced left ventricular systolic function
Revascularisation with coronary artery bypass grafting (CABG) is usually recommended over medical therapy for patients with CAD and LVSD37. However, for patients in whom surgical revascularisation is not feasible, PCI is recognised as a viable alternative revascularisation strategy3839. Left ventricular dysfunction remains an independent and strong predictor of in-hospital mortality40 and 30-day outcomes for PCI41. The adverse outcomes, death, stroke or myocardial infarction at 3 years, also remain significantly higher in patients with heart failure with reduced ejection fraction (HFrEF) irrespective of the revascularisation modality (PCI or CABG)42. As transient procedural ischaemia may result in a rapid destabilisation of narrowly compensated haemodynamics, operators should aim for a comprehensive approach to mitigate the risk during revascularisation procedures, a component of which may involve the use of a pLVAD. Haemodynamic stability and a reduction in hypotensive events have been achieved in patients with LV dysfunction undergoing PCI5. The IWG recommends using a pLVAD such as the Impella in extensive revascularisation procedures performed on patients with severe LVSD.
Patient comorbidities
High-risk patient characteristics are equally important for appropriate patient selection, as these characteristics may strongly affect outcomes. Patients with advanced age (>75 years), diabetes mellitus, advanced chronic kidney disease (glomerular filtration rate <30 ml/min/1.73 m2), surgical turndown and valvular heart disease are some of the important comorbidities that increase the risk for poor outcomes23536. Identification of elderly patients with significant other comorbidities and other clinical or technical characteristics is essential as they may benefit from protective devices to reduce the risk of complications. Similarly, patients with diabetes or advanced kidney disease, with a higher risk of more complex, diffusely calcified multivessel disease, may also benefit. The risk of periprocedural adverse events is higher in diabetics than non-diabetics, and diabetics pose a higher risk for contrast-induced AKI and the need for dialysis in patients with advanced kidney disease3643. Flaherty et al, in their retrospective single-centre study, analysed data from 230 patients undergoing high-risk PCI and demonstrated the renal protective effect of the Impella, where only 5.6% of supported patients developed AKI as compared to 27.8% of unsupported patients (p<0.001). Flaherty et al, in a prospective multicentre study, showed a lower incidence of AKI during high-risk PCI with the Impella as compared to their predicted AKI risk at baseline (4.9% vs 21.9%, respectively; p<0.0001)44. In another study, led by Westenfeld and colleagues, a retrospective analysis of 28 patients undergoing high-risk PCI with the support of the Impella versus the venoarterial (VA)-ECMO showed a lower incidence of contrast-induced AKI in patients supported by the Impella versus the VA-ECMO (12% vs 54%, respectively; p<0.05)45. Therefore, although the data are limited, the use of a pLVAD such as the Impella may prevent AKI in patients undergoing high-risk PCI.
Patients with severe aortic stenosis (AS) and mitral regurgitation also represent a group with a limited ability to tolerate haemodynamic embarrassment. A reduced capacity to augment myocardial oxygenation in response to stress is a physiological hallmark of AS, which may prove catastrophic in the event of transient myocardial ischaemia (decreased coronary blood flow) during high-risk PCI, leading to haemodynamic collapse171846. Similarly, in patients with decompensated mitral regurgitation, the increased wall stress may lead to a decreased myocardial oxygen supply, which in a state of transient but significant demand-supply mismatch during PCI may lead to haemodynamic collapse47. pLVADs like the Impella have been studied in these high-risk patients in small studies48, but larger studies are required to clarify the use of pLVADs in these subgroups of patients. Use of pLVADs may be warranted in patients with valvular diseases with concomitant LVSD as these patients have significantly higher rates of mortality353649.
Technical aspect – coronary anatomy and procedural characteristics IN unprotected left main disease
For patients with UPLM disease and a SYNTAX score >32 (high score), guidelines recommend the use of CABG over PCI. However, for patients with low-to-intermediate SYNTAX scores, the study comparing everolimus-eluting stents to bypass surgery for left main coronary artery disease (the EXCEL trial) showed comparable outcomes for PCI and CABG38395051. In patients who are at high surgical risk and are turned down for surgical revascularisation, high-risk PCI has emerged as a viable option. Moreover, having distal left main disease (bifurcation or a trifurcation lesion) is an independent predictor of worse outcomes, as compared to disease in the proximal part of the left main5253. Therefore, for patients with a SYNTAX score less than 32 but with distal left main disease (bifurcation or trifurcation), high-risk PCI can be considered. The use of pLVADs like the Impella has been adequately studied in this population in both trials and real-world data53254 and, thus, while managing UPLM stenosis or UPLM with distal disease (bifurcation or trifurcation) with a high SYNTAX score and concomitant LV dysfunction, the use of an Impella pLVAD may be beneficial.
Multivessel disease with a large area of myocardium at risk and requirement for complete revascularisation
Studies have shown that a complete anatomical revascularisation (i.e., residual SYNTAX scores of less than 8) in patients with multivessel coronary artery disease leads to lower long-term mortality rates and greater symptomatic improvement than an incomplete revascularisation, regardless of the revascularisation modality5556. In general, CABG is preferred over PCI in patients with diabetes and multivessel CAD with intermediate or high SYNTAX scores, whereas PCI is comparable to CABG in patients with MVD and low SYNTAX scores3839. Patients who are turned down for surgery for CABG can be considered for high-risk PCI.
Based on expert opinion, the IWG suggests that the presence of multivessel coronary artery disease alone, particularly with a low or moderate SYNTAX score does not justify the use of pLVAD support. The location of the lesion is very important in this context5758. If the multivessel disease involves the left anterior descending 1st septal (LAD–S1), or the left circumflex to 1st obtuse marginal (LCx to OM), or the right coronary artery to acute marginal (RCA to AM) supplying a large area of myocardium then these procedures can be considered as high-risk PCI, especially in presence of LVSD, and the use of an Impella pLVAD support is supported by the evidence and may be justified3259. The Impella has been shown to be effective in reducing post-PCI SYNTAX scores significantly325459 and, thus, may prove beneficial in improving outcomes in patients with MVD requiring PCI.
Only remaining conduit artery
The only remaining conduit artery (ORCA) is defined as a sole remaining conduit, native artery or bypass graft, with occlusion of the native and/or bypass supply to all remaining coronary territories35. The risk of revascularisation is high in these patients as any loss of flow in the ORCA can result in catastrophic ischaemia to the entire myocardium. The risk of flow loss is particularly high when the ORCA is a saphenous venous graft, given the elevated risk of the distal embolisation/slow-flow/no-reflow phenomenon that has been observed in vein graft interventions60. In cases requiring ORCA intervention or the use of a bypass graft ORCA as a conduit for recanalisation of the native coronary artery, the IWG felt that the use of an Impella pLVAD should be considered3536. In these cases, pLVAD use serves to both minimise the risk of haemodynamic compromise and facilitate multivessel revascularisation where technically feasible.
Procedural characteristics
Complex interventions and procedures can prolong the time of iatrogenic ischaemia leading to haemodynamic compromise. These procedures usually involve the extensive use of atherectomy devices, or retrograde recanalisation techniques in the case of chronic total occlusions. Atherectomy carries a risk of distal embolisation and no-reflow as a result of microvascular obstruction from atherectomised particles. Effectively, this may create a condition of transient large-territory ischaemia. In patients with concomitant LV dysfunction and/or multivessel CAD, even transient large-territory ischaemia (particularly when the atherectomised vessel supplies collateral flow to a chronically occluded vessel) can result in haemodynamic instability. Similarly, in chronic total occlusion (CTO)-PCI, where retrograde techniques are required, it virtually always results in some degree of transient ischaemia as blood flow through dominant collateral channels is obstructed by the equipment being advanced through them; this ischaemia can last several hours (the duration of complex CTO-PCI procedures) and may result in haemodynamic instability in the setting of pre-existent LV dysfunction3536. Accordingly, the IWG felt that the use of Impella pLVAD support in patients with moderate-to-severe LV dysfunction, which requires extensive or multiterritory atherectomy or the use of retrograde techniques for CTO, recanalisation should be considered.
Creating a decision-making tree for the use of haemodynamic support devices for India
There is a clear need to improve the current PCI risk-modelling framework in India. This may be achieved by collaborative data gathering and analysis, sharing of best practices, professional education, and skill enhancement. In the case of Impella pLVAD use, a quantifiable scoring system, the application of which could be studied across multiple institutions, was felt to be an important first step to optimising the use of this technology across India and South Asia. Accordingly, a decision-making tree and associated scoring system was developed by the IWG, using the principles and data discussed above (Figure 5). In general, the risk stratification and scoring system application for all high-risk PCI patients as defined above is as follows: all the members agreed that the decision-making process should start with careful preprocedural assessment by scoring systems such as the SYNTAX and Society of Thoracic Surgeons (STS) scores to identify coronary complexity and to define an optimal revascularisation strategy. Where high-risk PCI is recommended by the Heart Team, a careful review of the patient’s clinical history and most recent angiogram should be conducted to allow a score to be generated using the system shown in Figure 6. If a total score of more than 5 is obtained, then the use of Impella pLVAD support should be considered. For scores below 3, conventional PCI is suggested. For scores between 3 and 5, a careful risk-versus-benefit analysis should be carried out to allow for maximally informed, patient-centred decision-making. While there are existing algorithms and scoring systems for protected PCI proposed by experienced centres in the United States, such as the University of Washington (McCabe J. et al), our algorithm differs from those in several ways because it takes into consideration the anatomical and technical variables which exist in India, and these may also be relevant for other countries in the Asian subcontinent. Given the multiplicity of risk factors prevalent in India and, hence, the complex nature of coronary artery disease, we have extended the SYNTAX score cut-off point to 32 (as opposed to 22 in the Washington score) beyond which protected PCI may be considered. Low haemoglobin occurs in Indian patients due to a variety of reasons other than bleeding and, therefore, it was excluded from the risk assessment scoring system. Ejection fraction <25% gains a higher weightage at 2 points (instead of 1 point). In addition, we have considered advanced age as >75 years in our scoring system for protected PCI, as our elderly are frail with multiple comorbid risk factors.
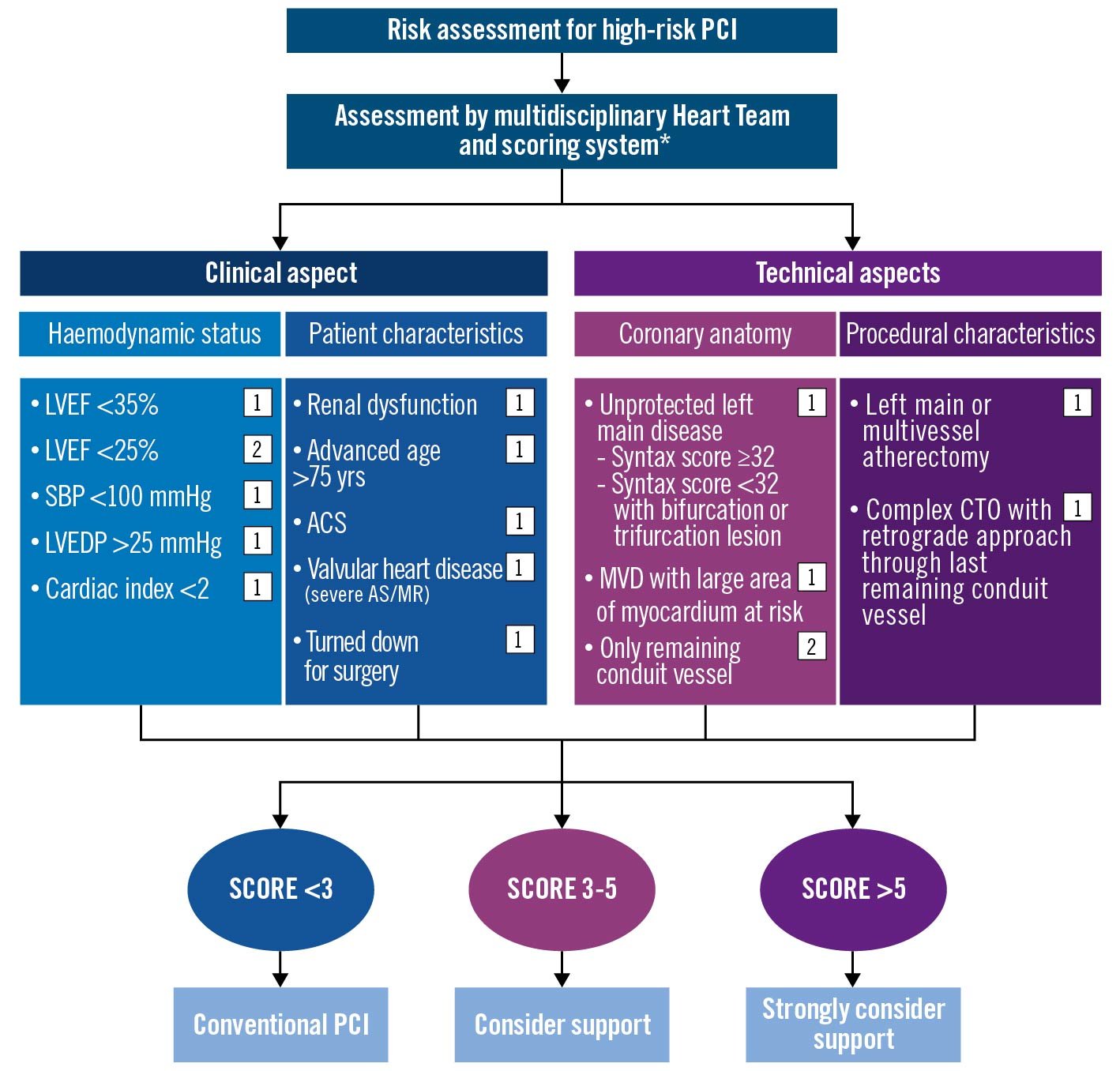
Figure 5. Proposed algorithmic risk assessment for protected PCI in India. *Society of Thoracic Surgeons. ACS: acute coronary syndrome; AS: aortic stenosis; CTO: chronic total occlusion; LVEDP: left ventricular end diastolic pressure; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVD: multivessel disease; PCI: percutaneous coronary intervention; SBP: systolic blood pressure. Created with BioRender.com
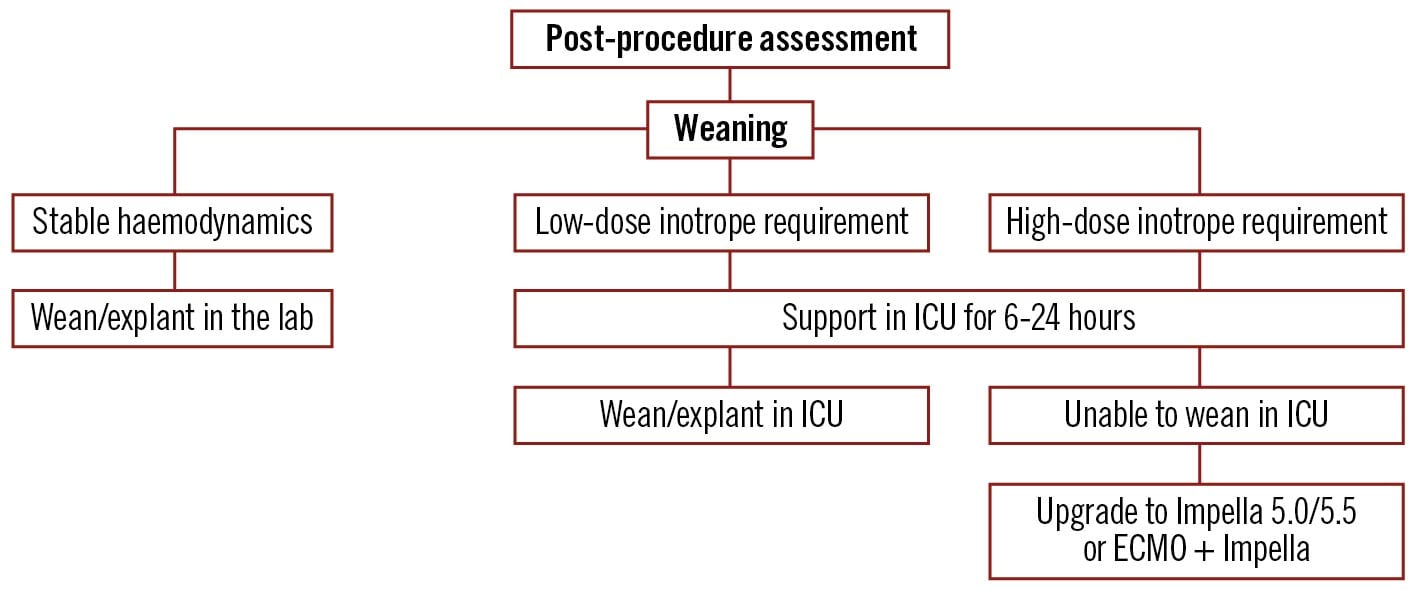
Figure 6. Algorithm for weaning of percutaneous left ventricular assist devices after completion of procedure. ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit. Created with BioRender.com
Conclusion and future directions
Although much progress has been made over the past two decades to improve the safety and effectiveness of PCI, these procedures still remain risky in those with severe LVSD and complex coronary anatomies. With the development of haemodynamic support devices such as the Impella pLVAD, there is an opportunity to decrease the complication rates and provide patients with safer, more complete revascularisation and, thus, optimised clinical outcomes. In India, where the changing demographics and worsening risk factors have led to an increase in the number of patients with complex coronary artery disease, a judicious but appropriate use of these devices is particularly important keeping in mind the added cost implications. The IWG Impella patient selection tool and the scoring system could help interventional cardiologists in India provide safer and more appropriate revascularisation to their highest-risk patients. However, a commitment to ongoing data collection and refinement of this decision-making tool through prospective evaluation of the Indian experience with the Impella pLVAD could be critical to outcome improvement for our high-risk patients in the years ahead.
Conflict of interest statement
R. Tayal received honoraria from Abiomed, Abbott, Shockwave Medical, and Edwards Lifesciences. S. Kalra received support and consulting fees from Abiomed, and Translumina Therapeutics; consulting fees from Abiomed, Cardiovascular Systems, Philips Healthcare, and Boston Scientific; support for attending meetings and/or travel from Abiomed, and Boston Scientific. He also held a leadership or fiduciary role in other board, society, committee or advocacy groups, paid or unpaid from Abiomed, and Boston Scientific. A. Seth is an Advisory Board Member of Abbott Vascular and SIS Medical; and received consulting fees from Boston Scientific, Medtronic, and Meril Lifesciences.The other authors have no conflicts of interest to declare.

