Florent Le Ven1, MD, PhD; Marie-Annick Clavel2, DVM, PhD; Philippe Pibarot2*, DVM, PhD
AsiaIntervention 2019;5:12-14, DOI: 10.4244/AIJV5I1A3
1. Hôpital Cavale Blanche, CHRU, Brest, France; 2. Institut Universitaire de Cardiologie et de Pneumologie de Québec/Québec Heart & Lung Institute, Laval University, Québec City, Québec, Canada
Recent studies have reported that low flow (LF), defined by a stroke volume index (SVi) <35 ml/m², is highly prevalent in patients with severe aortic stenosis (AS), and is associated with reduced survival1-5. This LF condition may occur in the context of either a reduced (i.e., classic LF) or preserved (i.e., paradoxical LF) left ventricular (LV) ejection fraction (LVEF), and is often associated with a low transvalvular pressure gradient6. Indeed, the pressure gradient is highly flow dependent and an LF state may thus be associated with a low gradient despite the presence of a severe AS. In patients undergoing transcatheter aortic valve replacement (TAVR), the presence of LF was one of the most powerful echocardiographic predictors of mortality and its impact was independent of LVEF or gradient7. Furthermore, the presence of LF early after the procedure was associated with a significant increase in subsequent mortality7.
In this issue of AsiaIntervention, Shirakawa et al8 present an elegant study in which they evaluated the preprocedural echocardiographic parameters that were associated with improved LV outflow following TAVR.
The authors showed that an increase in SVi following TAVR led to better one-year cardiac outcome (cardiac death and heart failure readmission). They also found that right ventricle fraction area change (RVFAC) and aortic regurgitation (AR) grade were associated with an increase in SVi after TAVR in multivariable analysis.
Prognostic impact of improvement in flow post TAVR
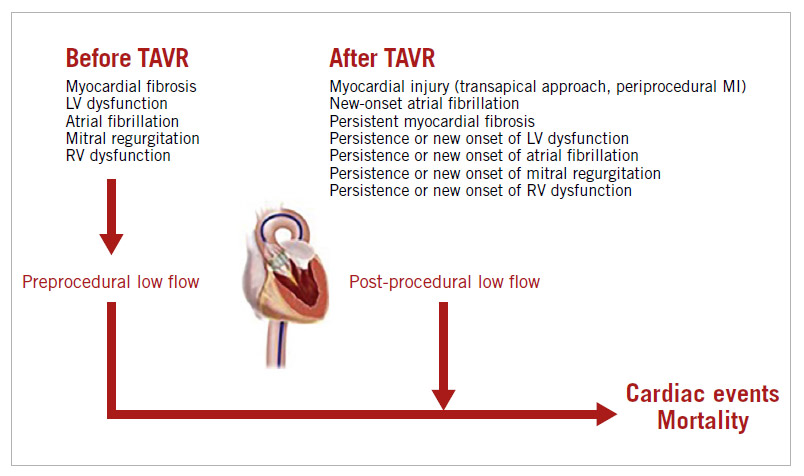
When compared to preprocedural flow, four situations may occur after TAVR: i) LF is present prior to and after the procedure (i.e., maintained LF); ii) LF is present prior to the procedure but flow (SVi) is normal after the procedure (i.e., normalised flow); iii) flow is normal prior to the procedure but low after the procedure (i.e., new-onset LF); and iv) flow is normal both before and after the procedure (i.e., maintained normal flow [NF]). Patients with persistent or new-onset LF seem to have an increased risk of mortality following TAVR compared with patients with normalised flow or maintained NF7,9. Persistent LF may reflect the presence of an advanced myocardial impairment with focal fibrosis, which is probably irreversible despite correction of the severe AS10, while new-onset LF may be related to periprocedural complications, such as myocardial injury (caused by the transapical approach for example) or atrial fibrillation7,11. Conversely, a normalised flow may happen in patients with diffuse myocardial fibrosis and/or very severe AS. The correction of the afterload excess by the TAVR procedure in those patients is generally beneficial and translates into improved survival. In the long term, the increase in SVi appears to be mainly related to an improvement in LVEF, regression of LV mass and concentric remodelling (which may, in turn, lead to improvement in LV diastolic function) (Figure 1). In their study, Shirakawa et al8 also found that increased SV, defined by the ratio of post-procedural SV/preprocedural SV >1, was associated with fewer cardiovascular events one year after TAVR. In addition, they found that, globally, SVi improves after TAVR, which corroborates the findings of previous studies7,12. Interestingly, the transapical approach did not appear to impact negatively on the improvement in SVi.
Predictors of SV improvement
In previous studies, the predictors of early post-procedural LF were lower preprocedural flow, the presence of atrial fibrillation, the use of the transapical approach, a lower post-procedural mean gradient and a lower baseline LVEF (Figure 1)7,9. Interestingly, Shirakawa et al found that patients harbouring a reduction in AR severity actually had an improvement in SVi. The underlying mechanism of this finding is unclear. Indeed, AR is typically associated with an increase in stroke volume. Accordingly, a previous study reported that moderate to severe AR on discharge echocardiography was associated with an increase in postoperative SVi9. However, the increase in flow related to AR is mainly observed in patients with chronic native AR with preserved LV systolic function. In the context of the TAVR population, there is a large proportion of patients with LV systolic dysfunction. The AR severity changes acutely with TAVR and is also associated with a concomitant reduction in the major LV afterload excess associated with severe AS. In this context, it is not necessarily surprising that an acute decrease in AR severity with TAVR is associated with improvement in LV outflow. Right ventricular dysfunction is associated with lower right and LV outflow and has been identified as an independent predictor of adverse outcomes in TAVR13 (Figure 1). In the present study, improvement in RVFAC was associated with an increase in SV following TAVR. We can hypothesise that, in addition to multiple complex factors such as LV/RV interrelations, a decrease in pulmonary artery pressure following a major reduction in LV afterload improvement may make a significant contribution to the improvement in LV outflow.
Clinical implications and future perspectives
This study by Shirakawa et al further demonstrates and emphasises that flow, as defined by SVi, is an important Dopplerechocardiographic parameter which should be assessed before as well as after intervention to evaluate early haemodynamic benefit and enhance long-term prognostication. LV outflow is an important overall surrogate marker of cardiac performance and prognosis (Figure 1). It thus appears logical and important to measure this parameter systematically in patients with AS. Despite its retrospective and observational design, this study also generates new hypotheses regarding the relationship between RV dysfunction, LF state and worse prognosis in patients with AS.
Figure 1. Predictors and impact on outcomes of low flow state prior to and after TAVR. MI: myocardial infarction
Conflict of interest statement
P. Pibarot has received funding from Edwards Lifesciences and Medtronic for echo core lab services in the field of TAVR with no personal compensation. The other authors have no conflicts of interest to declare.
References
1. Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-64.
2. Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235-43.
3. Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259-67.
4. Le Ven F, Freeman M, Webb J, Clavel MA, Wheeler M, Dumont É, Thompson C, De Larochellière R, Moss R, Doyle D, Ribeiro HB, Urena M, Nombela-Franco L, Rodés-Cabau J, Pibarot P. Impact of low flow on the outcome of high risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:782-8.
5. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of mortality and outcomes of therapy in low flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. 2013;127:2316-26.
6. Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845-53.
7. Le Ven F, Thébault C, Dahou A, Ribeiro HB, Capoulade R, Mahjoub H, Urena M, Nombela-Franco L, Allende Carrera R, Clavel MA, Dumont É, Dumesnil JG, De Larochellière R, Rodés- Cabau J, Pibarot P. Evolution and prognostic impact of low flow after transcatheter aortic valve replacement. Heart. 2015;101: 1196-203.
8. Shirakawa K, Itabashi Y, Tsuruta H, Endo J, Minakata Y, Hayashida K, Arai T, Yanagisawa R, Tanaka M, Shimizu H, Fukuda K, Murata M. Impact of preprocedural echocardiographic parameters on increased stroke volume after transcatheter aortic valve replacement. AsiaIntervention. 2019;5:72-80.
9. Anjan VY, Herrmann HC, Pibarot P, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani VH, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller C, Douglas PS, Leon MB. Evaluation of Flow After Transcatheter Aortic Valve Replacement in Patients With Low-Flow Aortic Stenosis: A Secondary Analysis of the PARTNER Randomized Clinical Trial. JAMA Cardiol. 2016;1:584-92.
10. Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577-84.
11. Ribeiro HB, Dahou A, Urena M, Carrasco JL, Mohammadi S, Doyle D, Le Ven F, Allende R, Amat-Santos I, Paradis JM, DeLarochellière R, Puri R, Abdul-Jawad Altisent O, Del Trigo M, Campelo-Parada F, Pibarot P, Dumont É, Rodés-Cabau J. Myocardial Injury After Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. Ann Thorac Surg. 2015; 99:2001-9.
12. Leon MB, Smith CR, Mack M, Miller C, Moses JW, Svensson LG, Tuzcu M, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PC, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-607.
13. Lindsay AC, Harron K, Jabbour RJ, Kanyal R, Snow TM, Sawhney P, Alpendurada F, Roughton M, Pennell DJ, Duncan A, Di Mario C, Davies SW, Mohiaddin RH, Moat NE. Prevalence and Prognostic Significance of Right Ventricular Systolic Dysfunction in Patients Undergoing Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv. 2016 Jul;9(7).
To download, please click below.